Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy
- PMID: 18332425
- PMCID: PMC2268833
- DOI: 10.1073/pnas.0800050105
Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy
Abstract
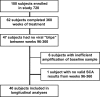
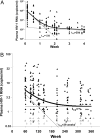
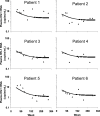
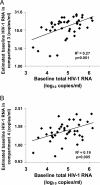
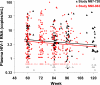
Residual viremia can be detected in most HIV-1-infected patients on antiretroviral therapy despite suppression of plasma RNA to <50 copies per ml, but the source and duration of this viremia is currently unknown. Therefore, we analyzed longitudinal plasma samples from 40 patients enrolled in the Abbott M97-720 trial at baseline (pretherapy) and weeks 60 to 384 by using an HIV-1 RNA assay with single-copy sensitivity. All patients were on therapy (lopinavir/ritonavir, stavudine, and lamivudine) with plasma HIV RNA <50 copies per ml by week 96 of the study and thereafter. Single-copy assay results revealed that 77% of the patient samples had detectable low-level viremia (>/=1 copy per ml), and all patients had at least one sample with detectable viremia. A nonlinear mixed effects model revealed a biphasic decline in plasma RNA levels occurring over weeks 60 to 384: an initial phase of decay with a half-life of 39 weeks and a subsequent phase with no perceptible decay. The level of pretherapy viremia extrapolated for each phase of decay was significantly correlated with total baseline viremia for each patient (R(2) = 0.27, P = 0.001 and R(2) = 0.19, P < 0.005, respectively), supporting a biological link between the extent of overall baseline viral infection and the infection of long-lived reservoirs. These data suggest that low-level persistent viremia appears to arise from at least two cell compartments, one in which viral production decays over time and a second in which viral production remains stable for at least 7 years.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia.PLoS Pathog. 2007 Apr;3(4):e46. doi: 10.1371/journal.ppat.0030046. PLoS Pathog. 2007. PMID: 17411338 Free PMC article. Clinical Trial.
-
Prevalence and predictive value of intermittent viremia with combination hiv therapy.JAMA. 2001 Jul 11;286(2):171-9. doi: 10.1001/jama.286.2.171. JAMA. 2001. PMID: 11448280
-
Time to HIV-1 RNA suppression below 5 copies/ml during first-line protease inhibitor-based antiretroviral treatment - any impact of residual viremia on treatment success?AIDS Rev. 2013 Oct-Dec;15(4):230-6. AIDS Rev. 2013. PMID: 24322383 Review.
-
Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine.J Infect Dis. 2005 Apr 1;191(7):1164-8. doi: 10.1086/428588. Epub 2005 Feb 28. J Infect Dis. 2005. PMID: 15747253
-
Advances in detection and monitoring of plasma viremia in HIV-infected individuals receiving antiretroviral therapy.Curr Opin HIV AIDS. 2013 Mar;8(2):87-92. doi: 10.1097/COH.0b013e32835d80af. Curr Opin HIV AIDS. 2013. PMID: 23314906 Review.
Cited by
-
Latent HIV-1 infection occurs in multiple subsets of hematopoietic progenitor cells and is reversed by NF-κB activation.J Virol. 2012 Sep;86(17):9337-50. doi: 10.1128/JVI.00895-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718820 Free PMC article.
-
HIV-1 Tat B-cell epitope vaccination was ineffectual in preventing viral rebound after ART cessation: HIV rebound with current ART appears to be due to infection with new endogenous founder virus and not to resurgence of pre-existing Tat-dependent viremia.Hum Vaccin Immunother. 2012 Oct;8(10):1425-30. doi: 10.4161/hv.21616. Epub 2012 Oct 1. Hum Vaccin Immunother. 2012. PMID: 23095869 Free PMC article. Clinical Trial.
-
Bridging HIV-1 cellular latency and clinical long-term non-progressor: an interactomic view.PLoS One. 2013;8(2):e55791. doi: 10.1371/journal.pone.0055791. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451031 Free PMC article.
-
Persistent HIV-1 replication during antiretroviral therapy.Curr Opin HIV AIDS. 2016 Jul;11(4):417-23. doi: 10.1097/COH.0000000000000287. Curr Opin HIV AIDS. 2016. PMID: 27078619 Free PMC article. Review.
-
Cell-Associated HIV-1 Unspliced-to-Multiply-Spliced RNA Ratio at 12 Weeks of ART Predicts Immune Reconstitution on Therapy.mBio. 2021 Mar 9;12(2):e00099-21. doi: 10.1128/mBio.00099-21. mBio. 2021. PMID: 33688002 Free PMC article.
References
-
- Dornadula G, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. J Am Med Assoc. 1999;282:1627–1632. - PubMed
-
- Havlir DV, et al. Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine. J Infect Dis. 2005;191:1164–1168. - PubMed
-
- Zhang L, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

