One-step affinity tag purification of full-length recombinant human AP-1 complexes from bacterial inclusion bodies using a polycistronic expression system
- PMID: 18329890
- PMCID: PMC2354920
- DOI: 10.1016/j.pep.2008.01.016
One-step affinity tag purification of full-length recombinant human AP-1 complexes from bacterial inclusion bodies using a polycistronic expression system
Abstract
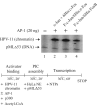
The AP-1 transcription factor is a dimeric protein complex formed primarily between Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra-1, Fra-2) family members. These distinct AP-1 complexes are expressed in many cell types and modulate target gene expression implicated in cell proliferation, differentiation, and stress responses. Although the importance of AP-1 has long been recognized, the biochemical characterization of AP-1 remains limited in part due to the difficulty in purifying full-length, reconstituted dimers with active DNA-binding and transcriptional activity. Using a combination of bacterial coexpression and epitope-tagging methods, we successfully purified all 12 heterodimers (3 Junx4 Fos) of full-length human AP-1 complexes as well as c-Jun/c-Jun, JunD/JunD, and c-Jun/JunD dimers from bacterial inclusion bodies using one-step nickel-NTA affinity tag purification following denaturation and renaturation of coexpressed AP-1 subunits. Coexpression of two constitutive components in a dimeric AP-1 complex helps stabilize the proteins when compared with individual protein expression in bacteria. Purified dimeric AP-1 complexes are functional in sequence-specific DNA binding, as illustrated by electrophoretic mobility shift assays and DNase I footprinting, and are also active in transcription with in vitro-reconstituted human papillomavirus (HPV) chromatin containing AP-1-binding sites in the native configuration of HPV nucleosomes. The availability of these recombinant full-length human AP-1 complexes has greatly facilitated mechanistic studies of AP-1-regulated gene transcription in many biological systems.
Figures



Similar articles
-
Occupancy and composition of proteins bound to the AP-1 sites in the glucocorticoid receptor and c-jun promoters after glucocorticoid treatment and in different cell types.Recept Signal Transduct. 1996;6(3-4):179-93. Recept Signal Transduct. 1996. PMID: 9259052
-
Binding site specificity and factor redundancy in activator protein-1-driven human papillomavirus chromatin-dependent transcription.J Biol Chem. 2011 Nov 25;286(47):40974-86. doi: 10.1074/jbc.M111.290874. Epub 2011 Sep 21. J Biol Chem. 2011. PMID: 21937452 Free PMC article.
-
Expression and purification of recombinant human c-Fos/c-Jun that is highly active in DNA binding and transcriptional activation in vitro.Nucleic Acids Res. 2001 Oct 15;29(20):E98. doi: 10.1093/nar/29.20.e98. Nucleic Acids Res. 2001. PMID: 11600717 Free PMC article.
-
Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors.Int J Biochem Cell Biol. 2006;38(7):1043-9. doi: 10.1016/j.biocel.2005.11.011. Epub 2005 Dec 20. Int J Biochem Cell Biol. 2006. PMID: 16423552 Review.
-
Neuronal expression of AP-1 proteins in excitotoxic-neurodegenerative disorders and following nerve fiber lesions.Prog Neurobiol. 1995 Nov-Dec;47(4-5):257-90. Prog Neurobiol. 1995. PMID: 26445738 Review.
Cited by
-
Involvement of Brd4 in different steps of the papillomavirus life cycle.Virus Res. 2017 Mar 2;231:76-82. doi: 10.1016/j.virusres.2016.12.006. Epub 2016 Dec 10. Virus Res. 2017. PMID: 27965149 Free PMC article. Review.
-
Opposing Effects of Zac1 and Curcumin on AP-1-Regulated Expressions of S100A7.PLoS One. 2015 Dec 3;10(12):e0144175. doi: 10.1371/journal.pone.0144175. eCollection 2015. PLoS One. 2015. PMID: 26633653 Free PMC article.
-
p53 sumoylation: mechanistic insights from reconstitution studies.Epigenetics. 2009 Oct 1;4(7):445-51. doi: 10.4161/epi.4.7.10030. Epub 2009 Oct 9. Epigenetics. 2009. PMID: 19838051 Free PMC article.
-
Systematic dissection of sequence features affecting binding specificity of a pioneer factor reveals binding synergy between FOXA1 and AP-1.Mol Cell. 2024 Aug 8;84(15):2838-2855.e10. doi: 10.1016/j.molcel.2024.06.022. Epub 2024 Jul 16. Mol Cell. 2024. PMID: 39019045
-
Chromatin adaptor Brd4 modulates E2 transcription activity and protein stability.J Biol Chem. 2009 Jan 30;284(5):2778-2786. doi: 10.1074/jbc.M805835200. Epub 2008 Nov 26. J Biol Chem. 2009. PMID: 19038968 Free PMC article.
References
-
- Eckert RL, Crish JF, Banks EB, Welter JF. The epidermis: genes on - genes off. J. Invest. Dermatol. 1997;109:501–509. - PubMed
-
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–136. - PubMed
-
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. - PubMed
-
- Gentz R, Rauscher FJ, Abate C, Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989;243:1695–1699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

