On helicases and other motor proteins
- PMID: 18329872
- PMCID: PMC2396192
- DOI: 10.1016/j.sbi.2008.01.007
On helicases and other motor proteins
Abstract
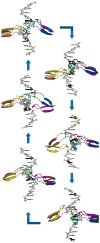
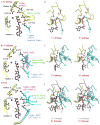
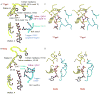
Helicases are molecular machines that utilize energy derived from ATP hydrolysis to move along nucleic acids and to separate base-paired nucleotides. The movement of the helicase can also be described as a stationary helicase that pumps nucleic acid. Recent structural data for the hexameric E1 helicase of papillomavirus in complex with single-stranded DNA and MgADP has provided a detailed atomic and mechanistic picture of its ATP-driven DNA translocation. The structural and mechanistic features of this helicase are compared with the hexameric helicase prototypes T7gp4 and SV40 T-antigen. The ATP-binding site architectures of these proteins are structurally similar to the sites of other prototypical ATP-driven motors such as F1-ATPase, suggesting related roles for the individual site residues in the ATPase activity.
Figures

Similar articles
-
A general model for nucleic acid helicases and their "coupling" within macromolecular machines.Cell. 2001 Jan 26;104(2):177-90. doi: 10.1016/s0092-8674(01)00203-3. Cell. 2001. PMID: 11207360 Review. No abstract available.
-
Running in reverse: the structural basis for translocation polarity in hexameric helicases.Cell. 2009 Oct 30;139(3):523-34. doi: 10.1016/j.cell.2009.08.043. Cell. 2009. PMID: 19879839 Free PMC article.
-
Structure and function of hexameric helicases.Annu Rev Biochem. 2000;69:651-97. doi: 10.1146/annurev.biochem.69.1.651. Annu Rev Biochem. 2000. PMID: 10966472 Review.
-
PcrA helicase, a prototype ATP-driven molecular motor.Structure. 2006 Sep;14(9):1345-53. doi: 10.1016/j.str.2006.06.017. Structure. 2006. PMID: 16962966
-
Helicases as nucleic acid unwinding machines.Nat Struct Biol. 2000 Jan;7(1):20-2. doi: 10.1038/71215. Nat Struct Biol. 2000. PMID: 10625420
Cited by
-
Fundamental Characteristics of AAA+ Protein Family Structure and Function.Archaea. 2016 Sep 14;2016:9294307. doi: 10.1155/2016/9294307. eCollection 2016. Archaea. 2016. PMID: 27703410 Free PMC article. Review.
-
Prp43p contains a processive helicase structural architecture with a specific regulatory domain.EMBO J. 2010 Jul 7;29(13):2194-204. doi: 10.1038/emboj.2010.102. Epub 2010 May 28. EMBO J. 2010. PMID: 20512115 Free PMC article.
-
Sequence-specific assembly of FtsK hexamers establishes directional translocation on DNA.Proc Natl Acad Sci U S A. 2010 Nov 23;107(47):20263-8. doi: 10.1073/pnas.1007518107. Epub 2010 Nov 3. Proc Natl Acad Sci U S A. 2010. PMID: 21048089 Free PMC article.
-
Exploring the Effect of Mechanical Anisotropy of Protein Structures in the Unfoldase Mechanism of AAA+ Molecular Machines.Nanomaterials (Basel). 2022 May 28;12(11):1849. doi: 10.3390/nano12111849. Nanomaterials (Basel). 2022. PMID: 35683705 Free PMC article.
-
Dynamic structural insights into the molecular mechanism of DNA unwinding by the bacteriophage T7 helicase.Nucleic Acids Res. 2020 Apr 6;48(6):3156-3164. doi: 10.1093/nar/gkaa057. Nucleic Acids Res. 2020. PMID: 32009150 Free PMC article.
References
-
- Ilyina TV, Gorbalenya AE, Koonin EV. Organization and evolution of bacterial and bacteriophage primase-helicase systems. J Mol Evol. 1992;34:351–357. - PubMed
-
- Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Current Opinion in Structural Biology. 1993;3:419–429. This manuscript outlines multiple conserved sequence motifs that define two helicase superfamilies.
-
- Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. This recent review article details the structure and mechanism of multiple superfamilies of helicases, defining precise details within the superfamilies and noting common features between them. - PubMed
-
- Mastrangelo IA, Hough PV, Wall JS, Dodson M, Dean FB, Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989;338:658–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

