Allosteric inhibition of the nonMyristoylated c-Abl tyrosine kinase by phosphopeptides derived from Abi1/Hssh3bp1
- PMID: 18328268
- PMCID: PMC2435598
- DOI: 10.1016/j.bbamcr.2008.01.028
Allosteric inhibition of the nonMyristoylated c-Abl tyrosine kinase by phosphopeptides derived from Abi1/Hssh3bp1
Abstract
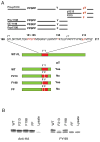
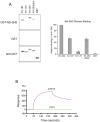
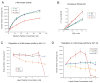
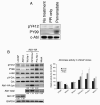
Here we report c-Abl kinase inhibition mediated by a phosphotyrosine located in trans in the c-Abl substrate, Abi1. The mechanism, which is pertinent to the nonmyristoylated c-Abl kinase, involves high affinity concurrent binding of the phosphotyrosine pY213 to the Abl SH2 domain and binding of a proximal PXXP motif to the Abl SH3 domain. Abi1 regulation of c-Abl in vivo appears to play a critical role, as demonstrated by inhibition of pY412 phosphorylation of the nonmyristoylated Abl by coexpression of Abi1. Pervanadate-induced c-Abl kinase activity was also reduced upon expression of the wild type Abi1 but not by expression of the Y213 to F213 mutant Abi1 in LNCaP cells, which are naturally deficient in the regulatory pY213. Our findings suggest a novel mechanism by which Abl kinase is regulated in cells.
Figures




Similar articles
-
Abi1/Hssh3bp1 pY213 links Abl kinase signaling to p85 regulatory subunit of PI-3 kinase in regulation of macropinocytosis in LNCaP cells.FEBS Lett. 2010 Aug 4;584(15):3279-86. doi: 10.1016/j.febslet.2010.06.029. Epub 2010 Jun 23. FEBS Lett. 2010. PMID: 20598684 Free PMC article.
-
Phosphorylation of c-Abl by protein kinase Pak2 regulates differential binding of ABI2 and CRK.Biochemistry. 2008 Jan 22;47(3):1094-104. doi: 10.1021/bi701533j. Epub 2007 Dec 28. Biochemistry. 2008. PMID: 18161990
-
Structure of a regulatory complex involving the Abl SH3 domain, the Crk SH2 domain, and a Crk-derived phosphopeptide.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):14053-8. doi: 10.1073/pnas.212518799. Epub 2002 Oct 16. Proc Natl Acad Sci U S A. 2002. PMID: 12384576 Free PMC article.
-
[Mechanism of c-Abl kinase activation by adaptor proteins].Seikagaku. 2006 Jun;78(6):521-5. Seikagaku. 2006. PMID: 16856564 Review. Japanese. No abstract available.
-
Regulation of the c-Abl and Bcr-Abl tyrosine kinases.Nat Rev Mol Cell Biol. 2004 Jan;5(1):33-44. doi: 10.1038/nrm1280. Nat Rev Mol Cell Biol. 2004. PMID: 14708008 Review.
Cited by
-
ABL tyrosine kinases: evolution of function, regulation, and specificity.Sci Signal. 2010 Sep 14;3(139):re6. doi: 10.1126/scisignal.3139re6. Sci Signal. 2010. PMID: 20841568 Free PMC article. Review.
-
Essential role for Abi1 in embryonic survival and WAVE2 complex integrity.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7022-7. doi: 10.1073/pnas.1016811108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482783 Free PMC article.
-
SH3 domains: modules of protein-protein interactions.Biophys Rev. 2013 Mar;5(1):29-39. doi: 10.1007/s12551-012-0081-z. Epub 2012 Jun 20. Biophys Rev. 2013. PMID: 28510178 Free PMC article. Review.
-
Structure and dynamic regulation of Abl kinases.J Biol Chem. 2013 Feb 22;288(8):5443-50. doi: 10.1074/jbc.R112.438382. Epub 2013 Jan 11. J Biol Chem. 2013. PMID: 23316053 Free PMC article. Review.
-
Crk and ABI1: binary molecular switches that regulate abl tyrosine kinase and signaling to the cytoskeleton.Genes Cancer. 2012 May;3(5-6):402-13. doi: 10.1177/1947601912460051. Genes Cancer. 2012. PMID: 23226578 Free PMC article.
References
-
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. - PubMed
-
- Woodring PJ, Hunter T, Wang JY. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116:2613–2626. - PubMed
-
- Druker BJ, Sawyers CL, Capdeville R, Ford JM, Baccarani M, Goldman JM. Chronic myelogenous leukemia. Hematology (Am Soc Hematol Educ Program) 2001:87–112. - PubMed
-
- Goldman JM, Melo JV. Chronic myeloid leukemia--advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451–1464. - PubMed
-
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ, Lydon NB. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

