Simultaneously targeting CD45 significantly increases cytotoxicity of the anti-CD33 immunoconjugate, gemtuzumab ozogamicin, against acute myeloid leukemia (AML) cells and improves survival of mice bearing human AML xenografts
- PMID: 18326813
- PMCID: PMC2343609
- DOI: 10.1182/blood-2008-01-133785
Simultaneously targeting CD45 significantly increases cytotoxicity of the anti-CD33 immunoconjugate, gemtuzumab ozogamicin, against acute myeloid leukemia (AML) cells and improves survival of mice bearing human AML xenografts
Abstract
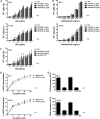
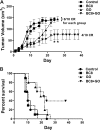
Targeting CD33 or CD45 is currently exploited for immunotherapy of acute myeloid leukemia (AML). Gemtuzumab ozogamicin (GO), an immunoconjugate of an anti-CD33 antibody that facilitates cellular uptake of a toxic calicheamicin-gamma(1) derivative, induces complete remissions in a subset of patients with AML. We herein tested whether simultaneous targeting of CD45 could improve GO cytotoxicity against AML cell lines and primary AML cells. We found that the anti-CD45 antibody, BC8, dose-dependently increased cytotoxicity induced by GO, and, to a lesser degree, free calicheamicin-gamma(1). BC8 promoted CD33 endocytosis, suggesting that its effect on GO cytotoxicity may be, at least partly, due to increased uptake and intracellular GO availability. Finally, compared with either agent alone, BC8 combined with GO resulted in marked tumor growth inhibition and superior survival rates of mice bearing human AML xenografts. These data suggest that further study of this antibody combination for clinical use in AML is warranted.
Figures
Similar articles
-
What happened to anti-CD33 therapy for acute myeloid leukemia?Curr Hematol Malig Rep. 2012 Mar;7(1):65-73. doi: 10.1007/s11899-011-0103-0. Curr Hematol Malig Rep. 2012. PMID: 22109628 Review.
-
CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance.Leukemia. 2005 Feb;19(2):176-82. doi: 10.1038/sj.leu.2403598. Leukemia. 2005. PMID: 15592433 Review.
-
In vitro experimental (211)At-anti-CD33 antibody therapy of leukaemia cells overcomes cellular resistance seen in vivo against gemtuzumab ozogamicin.Eur J Nucl Med Mol Imaging. 2010 May;37(5):851-61. doi: 10.1007/s00259-009-1356-x. Epub 2010 Jan 27. Eur J Nucl Med Mol Imaging. 2010. PMID: 20107790
-
Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia.Clin Cancer Res. 2005 Oct 1;11(19 Pt 2):7164s-7170s. doi: 10.1158/1078-0432.CCR-1004-0018. Clin Cancer Res. 2005. PMID: 16203817 Clinical Trial.
-
AKT signaling as a novel factor associated with in vitro resistance of human AML to gemtuzumab ozogamicin.PLoS One. 2013;8(1):e53518. doi: 10.1371/journal.pone.0053518. Epub 2013 Jan 8. PLoS One. 2013. PMID: 23320091 Free PMC article.
Cited by
-
Targeting human mitochondrial NAD(P)+-dependent malic enzyme (ME2) impairs energy metabolism and redox state and exhibits antileukemic activity in acute myeloid leukemia.Cell Oncol (Dordr). 2023 Oct;46(5):1301-1316. doi: 10.1007/s13402-023-00812-x. Epub 2023 Apr 20. Cell Oncol (Dordr). 2023. PMID: 37079187 Free PMC article.
-
Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin.Front Biosci (Landmark Ed). 2013 Jun 1;18(4):1311-34. doi: 10.2741/4181. Front Biosci (Landmark Ed). 2013. PMID: 23747885 Free PMC article. Review.
-
Siglecs as targets for therapy in immune-cell-mediated disease.Trends Pharmacol Sci. 2009 May;30(5):240-8. doi: 10.1016/j.tips.2009.02.005. Epub 2009 Apr 7. Trends Pharmacol Sci. 2009. PMID: 19359050 Free PMC article. Review.
-
In vivo human T cell engineering with enveloped delivery vehicles.Nat Biotechnol. 2024 Nov;42(11):1684-1692. doi: 10.1038/s41587-023-02085-z. Epub 2024 Jan 11. Nat Biotechnol. 2024. PMID: 38212493 Free PMC article.
-
What happened to anti-CD33 therapy for acute myeloid leukemia?Curr Hematol Malig Rep. 2012 Mar;7(1):65-73. doi: 10.1007/s11899-011-0103-0. Curr Hematol Malig Rep. 2012. PMID: 22109628 Review.
References
-
- Appelbaum FR. Immunobiologic therapies for myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17:653–661. - PubMed
-
- Abutalib SA, Tallman MS. Monoclonal antibodies for the treatment of acute myeloid leukemia. Curr Pharm Biotechnol. 2006;7:343–369. - PubMed
-
- Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19:176–182. - PubMed
-
- Pagano L, Fianchi L, Caira M, Rutella S, Leone G. The role of Gemtuzumab Ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene. 2007;26:3679–3690. - PubMed
-
- Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105:1295–1302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

