A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS
- PMID: 18322079
- PMCID: PMC6671170
- DOI: 10.1523/JNEUROSCI.4760-07.2008
A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS
Abstract
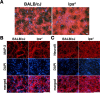
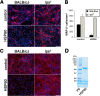
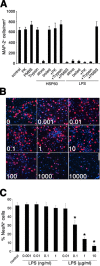
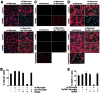
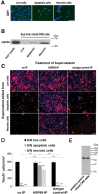
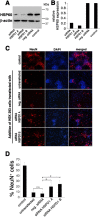
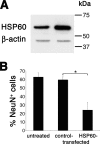
Infection, ischemia, trauma, and neoplasia elicit a similar inflammatory response in the CNS characterized by activation of microglia, the resident CNS monocyte. The molecular events leading from CNS injury to the activation of innate immunity is not well understood. We show here that the intracellular chaperone heat shock protein 60 (HSP60) serves as a signal of CNS injury by activating microglia through a toll-like receptor 4 (TLR4)-dependent and myeloid differentiation factor 88 (MyD88)-dependent pathway. HSP60 is released from CNS cells undergoing necrotic or apoptotic cell death and specifically binds to microglia. HSP60-induced synthesis of neurotoxic nitric oxide by microglia is dependent on TLR4. HSP60 induces extensive axonal loss and neuronal death in CNS cultures from wild-type but not TLR4 or MyD88 loss-of-function mutant mice. This is the first evidence of an endogenous molecular pathway common to many forms of neuronal injury that bidirectionally links CNS inflammation with neurodegeneration.
Figures


Similar articles
-
Intrathecal heat shock protein 60 mediates neurodegeneration and demyelination in the CNS through a TLR4- and MyD88-dependent pathway.Mol Neurodegener. 2015 Feb 26;10:5. doi: 10.1186/s13024-015-0003-1. Mol Neurodegener. 2015. PMID: 25887709 Free PMC article.
-
Microglial activation by Citrobacter koseri is mediated by TLR4- and MyD88-dependent pathways.J Immunol. 2009 Nov 1;183(9):5537-47. doi: 10.4049/jimmunol.0900083. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812209 Free PMC article.
-
Inhibiting expression of HSP60 and TLR4 attenuates paraquat-induced microglial inflammation.Chem Biol Interact. 2019 Feb 1;299:179-185. doi: 10.1016/j.cbi.2018.12.013. Epub 2018 Dec 22. Chem Biol Interact. 2019. PMID: 30584891
-
Modulating neuroinflammation in neurodegeneration-related dementia: can microglial toll-like receptors pull the plug?Metab Brain Dis. 2021 Jun;36(5):829-847. doi: 10.1007/s11011-021-00696-6. Epub 2021 Mar 11. Metab Brain Dis. 2021. PMID: 33704660 Review.
-
Toll-like receptor 4 in CNS pathologies.J Neurochem. 2010 Jul;114(1):13-27. doi: 10.1111/j.1471-4159.2010.06736.x. Epub 2010 Apr 6. J Neurochem. 2010. PMID: 20402965 Free PMC article. Review.
Cited by
-
Repetitive acute intermittent hypoxia does not promote generalized inflammatory gene expression in the rat CNS.Respir Physiol Neurobiol. 2015 Nov;218:1-10. doi: 10.1016/j.resp.2015.07.008. Epub 2015 Jul 26. Respir Physiol Neurobiol. 2015. PMID: 26213117 Free PMC article.
-
Neuroimmune Response in Ischemic Preconditioning.Neurotherapeutics. 2016 Oct;13(4):748-761. doi: 10.1007/s13311-016-0465-z. Neurotherapeutics. 2016. PMID: 27525700 Free PMC article. Review.
-
Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling.Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5697-707. doi: 10.1167/iovs.10-5407. Epub 2010 Jun 10. Invest Ophthalmol Vis Sci. 2010. PMID: 20538986 Free PMC article.
-
Stem Cell Therapies for Restorative Treatments of Central Nervous System Ischemia-Reperfusion Injury.Cell Mol Neurobiol. 2023 Mar;43(2):491-510. doi: 10.1007/s10571-022-01204-9. Epub 2022 Feb 7. Cell Mol Neurobiol. 2023. PMID: 35129759 Free PMC article. Review.
-
Heat shock proteins as biomarkers for the rapid detection of brain and spinal cord ischemia: a review and comparison to other methods of detection in thoracic aneurysm repair.Cell Stress Chaperones. 2011 Mar;16(2):119-31. doi: 10.1007/s12192-010-0224-8. Epub 2010 Aug 30. Cell Stress Chaperones. 2011. PMID: 20803353 Free PMC article. Review.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. - PubMed
-
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
