Nedd4-2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel
- PMID: 18322022
- PMCID: PMC2424110
- DOI: 10.1152/ajprenal.00339.2007
Nedd4-2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel
Abstract
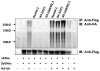
We previously reported the existence of multiple isoforms of human Nedd4-2 (Am J Physiol Renal Physiol 285: F916-F929, 2003). When overexpressed in M-1 collecting duct epithelia, full-length Nedd4-2 (Nedd4-2), Nedd4-2 lacking the NH(2)-terminal C2 domain (Nedd4-2DeltaC2), and Nedd4-2 lacking WW domains 2 and 3 (Nedd4-2DeltaWW2,3) variably reduce benzamil-sensitive Na(+) transport. We investigated the effect of each of the Nedd4-2 isoforms on cell surface expression and ubiquitination of ENaC subunits. We find that alphaENaC when transfected alone or with beta and gammaENaC is expressed at the cell surface and this membrane expression is variably reduced by coexpression with each of the Nedd4-2 isoforms. Nedd4-2 reduces the half-life of ENaC subunits and enhances the ubiquitination of alpha, beta, and gammaENaC subunits when expressed alone or together suggesting that each subunit is a target for Nedd4-2-mediated ubiquitination. As has been reported recently, we confirm that the surface-expressed pool of ENaC is multi-ubiquitinated. Inhibitors of the proteasome increase ubiquitination of ENaC subunits and stimulate Na(+) transport in M-1 cells consistent with a role for the ubiquitin-proteasome pathway in regulating Na(+) transport in the collecting duct.
Figures
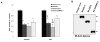


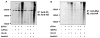



Similar articles
-
Nedd4-2 isoforms differentially associate with ENaC and regulate its activity.Am J Physiol Renal Physiol. 2005 Aug;289(2):F334-46. doi: 10.1152/ajprenal.00394.2004. Epub 2005 Apr 5. Am J Physiol Renal Physiol. 2005. PMID: 15814530
-
Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC.J Biol Chem. 2007 Jul 13;282(28):20207-12. doi: 10.1074/jbc.M611329200. Epub 2007 May 14. J Biol Chem. 2007. PMID: 17502380
-
Functional regulation of the epithelial Na+ channel by IkappaB kinase-beta occurs via phosphorylation of the ubiquitin ligase Nedd4-2.J Biol Chem. 2009 Jan 2;284(1):150-157. doi: 10.1074/jbc.M807358200. Epub 2008 Nov 3. J Biol Chem. 2009. PMID: 18981174 Free PMC article.
-
Regulation of epithelial sodium channel trafficking by ubiquitination.Proc Am Thorac Soc. 2010 Feb;7(1):54-64. doi: 10.1513/pats.200909-096JS. Proc Am Thorac Soc. 2010. PMID: 20160149 Free PMC article. Review.
-
Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway.Am J Physiol Renal Physiol. 2006 Jun;290(6):F1285-94. doi: 10.1152/ajprenal.00432.2005. Am J Physiol Renal Physiol. 2006. PMID: 16682484 Review.
Cited by
-
Salt-induced hypertension in a mouse model of Liddle syndrome is mediated by epithelial sodium channels in the brain.Hypertension. 2012 Sep;60(3):691-6. doi: 10.1161/HYPERTENSIONAHA.112.193045. Epub 2012 Jul 16. Hypertension. 2012. PMID: 22802227 Free PMC article.
-
NEDD4-2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins.Gene. 2015 Feb 15;557(1):1-10. doi: 10.1016/j.gene.2014.11.051. Epub 2014 Nov 26. Gene. 2015. PMID: 25433090 Free PMC article. Review.
-
An evolutionarily conserved N-terminal Sgk1 variant with enhanced stability and improved function.Am J Physiol Renal Physiol. 2008 Nov;295(5):F1440-8. doi: 10.1152/ajprenal.90239.2008. Epub 2008 Aug 27. Am J Physiol Renal Physiol. 2008. PMID: 18753299 Free PMC article.
-
Enhanced expression of epithelial sodium channels causes salt-induced hypertension in mice through inhibition of the α2-isoform of Na+, K+-ATPase.Physiol Rep. 2015 May;3(5):e12383. doi: 10.14814/phy2.12383. Physiol Rep. 2015. PMID: 25991719 Free PMC article.
-
Nedd4-2 interacts with occludin to inhibit tight junction formation and enhance paracellular conductance in collecting duct epithelia.Am J Physiol Renal Physiol. 2010 Aug;299(2):F436-44. doi: 10.1152/ajprenal.00674.2009. Epub 2010 May 26. Am J Physiol Renal Physiol. 2010. PMID: 20504882 Free PMC article.
References
-
- Ardley HC, Robinson PA. E3 ubiquitin ligases. Essays Biochem. 2005;41:15–30. - PubMed
-
- Asher C, Sinha I, Garty H. Characterization of the interactions between Nedd4-2, ENaC, and sgk-1 using surface plasmon resonance. Biochim Biophys Acta. 2003;1612:59–64. - PubMed
-
- Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. SGK1 regulates ubiquitin ligase Nedd4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005:me.2005–0193. - PubMed
-
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated Kinase Inhibits the Epithelial Na+ Channel through Functional Regulation of the Ubiquitin Ligase Nedd4-2. J Biol Chem. 2006;281:26159–26169. - PubMed
-
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

