Inhibitors of strand transfer that prevent integration and inhibit human T-cell leukemia virus type 1 early replication
- PMID: 18316517
- PMCID: PMC2565912
- DOI: 10.1128/AAC.01361-07
Inhibitors of strand transfer that prevent integration and inhibit human T-cell leukemia virus type 1 early replication
Abstract
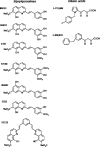
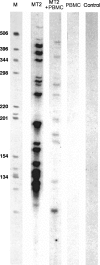
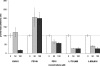
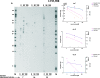
The replication of the retrovirus human T-cell leukemia virus type 1 (HTLV-1) is linked to the development of lymphoid malignancies and inflammatory diseases. Data from in vitro, ex vivo, and in vivo studies have revealed that no specific treatment can prevent or block HTLV-1 replication and therefore that there is no therapy for the prevention and/or treatment of HTLV-1-associated diseases in infected individuals. HTLV-1 and human immunodeficiency virus type 1 (HIV-1) integrases, the enzymes that specifically catalyze the integration of these retroviruses in host cell DNA, share important structural properties, suggesting that compounds that inhibit HIV-1 integration could also inhibit HTLV-1 integration. We developed quantitative assays to test, in vitro and ex vivo, the efficiencies of styrylquinolines and diketo acids, the two main classes of HIV-1 integrase inhibitors. The compounds were tested in vitro in an HTLV-1 strand-transfer reaction and ex vivo by infection of fresh peripheral blood lymphocytes with lethally irradiated HTLV-1-positive cells. In vitro, four styrylquinoline compounds and two diketo acid compounds significantly inhibited HTLV-1 integration in a dose-dependent manner. All compounds active in vitro decreased cell proliferation ex vivo, although at low concentrations; they also dramatically decreased both normalized proviral loads and the number of integration events during experimental ex vivo primary infection. Accordingly, diketo acids and styrylquinolines are the first drugs that produce a specific negative effect on HTLV-1 replication in vitro and ex vivo, suggesting their potential efficiency for the prevention and treatment of HTLV-1-associated diseases.
Figures
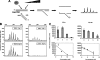
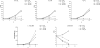

Similar articles
-
Integrase inhibitors effective against human T-cell leukemia virus type 1.Antimicrob Agents Chemother. 2011 May;55(5):2011-7. doi: 10.1128/AAC.01413-10. Epub 2011 Feb 22. Antimicrob Agents Chemother. 2011. PMID: 21343468 Free PMC article.
-
Drug targets in human T-lymphotropic virus type 1 (HTLV-1) infection.Infect Disord Drug Targets. 2009 Apr;9(2):159-71. doi: 10.2174/187152609787847686. Infect Disord Drug Targets. 2009. PMID: 19275704 Review.
-
Antiretroviral therapy promotes an inflammatory-like pattern of human T-cell lymphotropic virus type 1 (HTLV-1) replication in human immunodeficiency virus type 1/HTLV-1 co-infected individuals.J Gen Virol. 2013 Apr;94(Pt 4):753-757. doi: 10.1099/vir.0.048348-0. Epub 2012 Dec 12. J Gen Virol. 2013. PMID: 23239567
-
Basis of HTLV type 1 target site selection.AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1653-9. doi: 10.1089/08892220050193100. AIDS Res Hum Retroviruses. 2000. PMID: 11080806
-
HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection.Semin Cancer Biol. 2014 Jun;26:89-98. doi: 10.1016/j.semcancer.2013.11.003. Epub 2013 Dec 6. Semin Cancer Biol. 2014. PMID: 24316494 Free PMC article. Review.
Cited by
-
Activation of PERK-ATF4-CHOP pathway as a novel therapeutic approach for efficient elimination of HTLV-1-infected cells.Blood Adv. 2020 May 12;4(9):1845-1858. doi: 10.1182/bloodadvances.2019001139. Blood Adv. 2020. PMID: 32369565 Free PMC article.
-
Antiretroviral Therapy in HTLV-1 Infection: An Updated Overview.Pathogens. 2020 May 1;9(5):342. doi: 10.3390/pathogens9050342. Pathogens. 2020. PMID: 32369988 Free PMC article. Review.
-
Structural and functional insights into foamy viral integrase.Viruses. 2013 Jul 18;5(7):1850-66. doi: 10.3390/v5071850. Viruses. 2013. PMID: 23872492 Free PMC article. Review.
-
HIV‑1 integrase inhibitors targeting various DDE transposases: Retroviral integration versus RAG‑mediated recombination (Review).Mol Med Rep. 2019 Dec;20(6):4749-4762. doi: 10.3892/mmr.2019.10777. Epub 2019 Oct 30. Mol Med Rep. 2019. PMID: 31702817 Free PMC article. Review.
-
Recent Updates on Viral Oncogenesis: Available Preventive and Therapeutic Entities.Mol Pharm. 2023 Aug 7;20(8):3698-3740. doi: 10.1021/acs.molpharmaceut.2c01080. Epub 2023 Jul 24. Mol Pharm. 2023. PMID: 37486263 Free PMC article. Review.
References
-
- Bazarbachi, A., D. Ghez, Y. Lepelletier, R. Nasr, H. de The, M. E. El-Sabban, and O. Hermine. 2004. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 5:664-672. - PubMed
-
- Bazarbachi, A., R. Nasr, M. E. El-Sabban, A. Mahe, R. Mahieux, A. Gessain, N. Darwiche, G. Dbaibo, J. Kersual, Y. Zermati, L. Dianoux, M. K. Chelbi-Alix, H. de The, and O. Hermine. 2000. Evidence against a direct cytotoxic effect of alpha interferon and zidovudine in HTLV-I associated adult T cell leukemia/lymphoma. Leukemia 14:716-721. - PubMed
-
- Benard, C., F. Zouhiri, M. Normand-Bayle, M. Danet, D. Desmaele, H. Leh, J. F. Mouscadet, G. Mbemba, C. M. Thomas, S. Bonnenfant, M. Le Bret, and J. d'Angelo. 2004. Linker-modified quinoline derivatives targeting HIV-1 integrase: synthesis and biological activity. Bioorg. Med. Chem. Lett. 14:2473-2476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

