Gephyrin interacts with the glutamate receptor interacting protein 1 isoforms at GABAergic synapses
- PMID: 18315564
- PMCID: PMC2891215
- DOI: 10.1111/j.1471-4159.2008.05311.x
Gephyrin interacts with the glutamate receptor interacting protein 1 isoforms at GABAergic synapses
Abstract
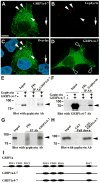
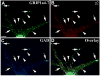
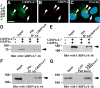
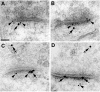
We have previously shown that the glutamate receptor interacting protein 1 (GRIP1) splice forms GRIP1a/b and GRIP1c4-7 are present at the GABAergic post-synaptic complex. Nevertheless, the role that these GRIP1 protein isoforms play at the GABAergic post-synaptic complex is not known. We are now showing that GRIP1c4-7 and GRIP1a/b interact with gephyrin, the main post-synaptic scaffold protein of GABAergic and glycinergic synapses. Gephyrin coprecipitates with GRIP1c4-7 or GRIP1a/b from rat brain extracts and from extracts of human embryonic kidney 293 cells that have been cotransfected with gephyrin and one of the GRIP1 protein isoforms. Moreover, purified gephyrin binds to purified GRIP1c4-7 or GRIP1a/b, indicating that gephyrin directly interacts with the common region of these GRIP1 proteins, which includes PDZ domains 4-7. An engineered deletion construct of GRIP1a/b (GRIP1a4-7), which both contains the aforementioned common region and binds to gephyrin, targets to the post-synaptic GABAergic complex of transfected cultured hippocampal neurons. In these hippocampal cultures, endogenous gephyrin colocalizes with endogenous GRIP1c4-7 and GRIP1a/b in over 90% of the GABAergic synapses. Double-labeling electron microscopy immunogold reveals that in the rat brain GRIP1c4-7 and GRIP1a/b colocalize with gephyrin at the post-synaptic complex of individual synapses. These results indicate that GRIP1c4-7 and GRIP1a/b colocalize and interact with gephyrin at the GABAergic post-synaptic complex and suggest that this interaction plays a role in GABAergic synaptic function.
Figures



Similar articles
-
GRIP1 in GABAergic synapses.J Comp Neurol. 2005 Jul 18;488(1):11-27. doi: 10.1002/cne.20566. J Comp Neurol. 2005. PMID: 15912503
-
A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain.J Biol Chem. 2004 Sep 10;279(37):38978-90. doi: 10.1074/jbc.M405786200. Epub 2004 Jun 29. J Biol Chem. 2004. PMID: 15226318
-
Identification and characterization of two novel splice forms of GRIP1 in the rat brain.J Neurochem. 2006 May;97(3):884-98. doi: 10.1111/j.1471-4159.2006.03795.x. Epub 2006 Mar 15. J Neurochem. 2006. PMID: 16539648
-
Gephyrin: where do we stand, where do we go?Trends Neurosci. 2008 May;31(5):257-64. doi: 10.1016/j.tins.2008.02.006. Epub 2008 Apr 9. Trends Neurosci. 2008. PMID: 18403029 Review.
-
Gephyrin: a master regulator of neuronal function?Nat Rev Neurosci. 2014 Mar;15(3):141-56. doi: 10.1038/nrn3670. Nat Rev Neurosci. 2014. PMID: 24552784 Review.
Cited by
-
Molecular and functional interaction between protocadherin-γC5 and GABAA receptors.J Neurosci. 2012 Aug 22;32(34):11780-97. doi: 10.1523/JNEUROSCI.0969-12.2012. J Neurosci. 2012. PMID: 22915120 Free PMC article.
-
Differential regulation of the postsynaptic clustering of γ-aminobutyric acid type A (GABAA) receptors by collybistin isoforms.J Biol Chem. 2011 Jun 24;286(25):22456-68. doi: 10.1074/jbc.M111.236190. Epub 2011 May 3. J Biol Chem. 2011. PMID: 21540179 Free PMC article.
-
Gain-of-function glutamate receptor interacting protein 1 variants alter GluA2 recycling and surface distribution in patients with autism.Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):4920-5. doi: 10.1073/pnas.1102233108. Epub 2011 Mar 7. Proc Natl Acad Sci U S A. 2011. PMID: 21383172 Free PMC article.
-
NMDA receptors in GABAergic synapses during postnatal development.PLoS One. 2012;7(5):e37753. doi: 10.1371/journal.pone.0037753. Epub 2012 May 25. PLoS One. 2012. PMID: 22662211 Free PMC article.
-
Recruitment of Plasma Membrane GABA-A Receptors by Submembranous Gephyrin/Collybistin Clusters.Cell Mol Neurobiol. 2022 Jul;42(5):1585-1604. doi: 10.1007/s10571-021-01050-1. Epub 2021 Feb 5. Cell Mol Neurobiol. 2022. PMID: 33547626
References
-
- Bohlhalter S, Mohler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Res. 1994;642:59–69. - PubMed
-
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. - PubMed
-
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004a;279:38978–38990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

