Transcription factors sp1 and sp3 regulate expression of human extracellular superoxide dismutase in lung fibroblasts
- PMID: 18314536
- PMCID: PMC2542458
- DOI: 10.1165/rcmb.2007-0378OC
Transcription factors sp1 and sp3 regulate expression of human extracellular superoxide dismutase in lung fibroblasts
Abstract
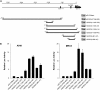
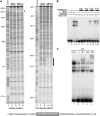
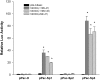
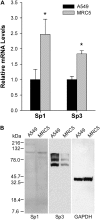
The molecular mechanisms that govern the transcription of human extracellular superoxide dismutase (EC-SOD), the major extracellular antioxidant enzyme, are largely unknown. To elucidate the mechanisms involved in human EC-SOD gene regulation and expression, we localized multiple transcription start sites to a finite region located 3.9 kb upstream of the ATG initiation codon. Within this segment, we subcloned a 2.7-kb fragment upstream of a luciferase reporter gene; the resulting construct exhibited strong in vivo promoter activity in two lung-derived cell lines. Deletion analysis of the EC-SOD 5'-flanking sequences identified a minimal 0.3-kb region that had strong basal promoter activity. Computer sequence analysis revealed a putative Sp1-like binding site within the EC-SOD proximal promoter region that lacked a TATA-box and showed a high frequency of GC nucleotides. Binding of Sp1 and Sp3 transcription factors to the EC-SOD promoter was confirmed by DNase I footprint analysis, electophoretic mobility shift assay, and competition and supershift assays. Cotransfection of the EC-SOD promoter-luciferase reporter constructs with plasmids encoding Sp1 and Sp3 into Sp-deficient insect SL2 cells showed strong activation of luciferase gene expression. The occupancy of the EC-SOD promoter by Sp1/Sp3 and RNA polymerase II in vivo was determined by chromatin immunoprecipitation assay and correlated well with levels of EC-SOD expression in lung epithelial cells (A549) and pulmonary fibroblasts (MRC5). Collectively, our results demonstrate the important role Sp1 and Sp3 plays in regulating the expression of human EC-SOD in the lung.
Figures

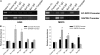
Similar articles
-
Sp1 and Sp3 transcription factors mediate trichostatin A-induced and basal expression of extracellular superoxide dismutase.Free Radic Biol Med. 2004 Oct 15;37(8):1256-71. doi: 10.1016/j.freeradbiomed.2004.06.022. Free Radic Biol Med. 2004. PMID: 15451065
-
Cloning and characterization of the human heparanase-1 (HPR1) gene promoter: role of GA-binding protein and Sp1 in regulating HPR1 basal promoter activity.J Biol Chem. 2002 Mar 15;277(11):8989-98. doi: 10.1074/jbc.M105682200. Epub 2002 Jan 4. J Biol Chem. 2002. PMID: 11779847
-
Sp1/Sp3 and DNA-methylation contribute to basal transcriptional activation of human podoplanin in MG63 versus Saos-2 osteoblastic cells.BMC Mol Biol. 2007 Mar 7;8:20. doi: 10.1186/1471-2199-8-20. BMC Mol Biol. 2007. PMID: 17343736 Free PMC article.
-
The human receptor tyrosine kinase Axl gene--promoter characterization and regulation of constitutive expression by Sp1, Sp3 and CpG methylation.Biosci Rep. 2008 Jun;28(3):161-76. doi: 10.1042/BSR20080046. Biosci Rep. 2008. PMID: 18522535
-
Sp1 and Sp3 regulate basal transcription of the human CYP2F1 gene.Drug Metab Dispos. 2005 Aug;33(8):1244-53. doi: 10.1124/dmd.105.004069. Epub 2005 Apr 28. Drug Metab Dispos. 2005. PMID: 15860659
Cited by
-
Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis.Mol Cancer Res. 2012 Jan;10(1):40-51. doi: 10.1158/1541-7786.MCR-11-0501. Epub 2011 Nov 7. Mol Cancer Res. 2012. PMID: 22064654 Free PMC article.
-
Tumor necrosis factor-α decreases EC-SOD expression through DNA methylation.J Clin Biochem Nutr. 2017 May;60(3):169-175. doi: 10.3164/jcbn.16-111. Epub 2017 Apr 7. J Clin Biochem Nutr. 2017. PMID: 28584398 Free PMC article.
-
Trefoil factor family 2 expression inhibits gastric cancer cell growth and invasion in vitro via interactions with the transcription factor Sp3.Int J Mol Med. 2016 Nov;38(5):1474-1480. doi: 10.3892/ijmm.2016.2739. Epub 2016 Sep 19. Int J Mol Med. 2016. PMID: 27668303 Free PMC article.
-
Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients.Mol Vis. 2009 Dec 10;15:2663-72. Mol Vis. 2009. PMID: 20011081 Free PMC article.
-
Regulation of superoxide dismutase genes: implications in disease.Free Radic Biol Med. 2009 Aug 15;47(4):344-56. doi: 10.1016/j.freeradbiomed.2009.05.018. Epub 2009 May 25. Free Radic Biol Med. 2009. PMID: 19477268 Free PMC article. Review.
References
-
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 2002;33:337–349. - PubMed
-
- Noor R, Mittal S, Iqbal J. Superoxide dismutase–applications and relevance to human diseases. Med Sci Monit 2002;8:RA210–RA215. - PubMed
-
- Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 2003;35:236–256. - PubMed
-
- Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med 2003;167:400–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

