Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation
- PMID: 18305110
- PMCID: PMC2442318
- DOI: 10.1074/jbc.M800342200
Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation
Abstract
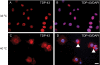
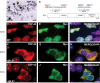
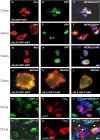
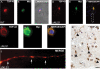
TAR DNA-binding protein 43 (TDP-43) is the disease protein in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) and amyotrophic lateral sclerosis (ALS). Although normal TDP-43 is a nuclear protein, pathological TDP-43 is redistributed and sequestered as insoluble aggregates in neuronal nuclei, perikarya, and neurites. Here we recapitulate these pathological phenotypes in cultured cells by altering endogenous TDP-43 nuclear trafficking and by expressing mutants with defective nuclear localization (TDP-43-DeltaNLS) or nuclear export signals (TDP-43-DeltaNES). Restricting endogenous cytoplasmic TDP-43 from entering the nucleus or preventing its exit out of the nucleus resulted in TDP-43 aggregate formation. TDP-43-DeltaNLS accumulates as insoluble cytoplasmic aggregates and sequesters endogenous TDP-43, thereby depleting normal nuclear TDP-43, whereas TDP-43-DeltaNES forms insoluble nuclear aggregates with endogenous TDP-43. Mutant forms of TDP-43 also replicate the biochemical profile of pathological TDP-43 in FTLD-U/ALS. Thus, FTLD-U/ALS pathogenesis may be linked mechanistically to deleterious perturbations of nuclear trafficking and solubility of TDP-43.
Figures


Similar articles
-
A90V TDP-43 variant results in the aberrant localization of TDP-43 in vitro.FEBS Lett. 2008 Jun 25;582(15):2252-6. doi: 10.1016/j.febslet.2008.05.024. Epub 2008 May 27. FEBS Lett. 2008. PMID: 18505686 Free PMC article.
-
Developmentally Regulated RNA-binding Protein 1 (Drb1)/RNA-binding Motif Protein 45 (RBM45), a Nuclear-Cytoplasmic Trafficking Protein, Forms TAR DNA-binding Protein 43 (TDP-43)-mediated Cytoplasmic Aggregates.J Biol Chem. 2016 Jul 15;291(29):14996-5007. doi: 10.1074/jbc.M115.712232. Epub 2016 May 12. J Biol Chem. 2016. PMID: 27226551 Free PMC article.
-
Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies.J Biol Chem. 2009 Mar 27;284(13):8516-24. doi: 10.1074/jbc.M809462200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164285 Free PMC article.
-
[The molecular mechanisms of intracellular TDP-43 aggregates].Brain Nerve. 2009 Nov;61(11):1292-300. Brain Nerve. 2009. PMID: 19938686 Review. Japanese.
-
[TDP-43 proteinopathies, toward understanding of the molecular pathogenesis].Rinsho Shinkeigaku. 2009 Nov;49(11):783-5. doi: 10.5692/clinicalneurol.49.783. Rinsho Shinkeigaku. 2009. PMID: 20030209 Review. Japanese.
Cited by
-
The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation.Hum Mol Genet. 2013 Aug 1;22(15):3112-22. doi: 10.1093/hmg/ddt166. Epub 2013 Apr 10. Hum Mol Genet. 2013. PMID: 23575225 Free PMC article.
-
Selective forelimb impairment in rats expressing a pathological TDP-43 25 kDa C-terminal fragment to mimic amyotrophic lateral sclerosis.Mol Ther. 2013 Jul;21(7):1324-34. doi: 10.1038/mt.2013.88. Epub 2013 May 21. Mol Ther. 2013. PMID: 23689600 Free PMC article.
-
Poly(ADP-Ribose) Prevents Pathological Phase Separation of TDP-43 by Promoting Liquid Demixing and Stress Granule Localization.Mol Cell. 2018 Sep 6;71(5):703-717.e9. doi: 10.1016/j.molcel.2018.07.002. Epub 2018 Aug 9. Mol Cell. 2018. PMID: 30100264 Free PMC article.
-
Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis.J Neurosci. 2010 Jan 13;30(2):639-49. doi: 10.1523/JNEUROSCI.4988-09.2010. J Neurosci. 2010. PMID: 20071528 Free PMC article.
-
Effect of TDP43-CTFs35 on Brain Endothelial Cell Functions in Cerebral Ischemic Injury.Mol Neurobiol. 2022 Jul;59(7):4593-4611. doi: 10.1007/s12035-022-02869-5. Epub 2022 May 18. Mol Neurobiol. 2022. PMID: 35581521
References
-
- Ayala, Y. M., Pantano, S., D'Ambrogio, A., Buratti, E., Brindisi, A., Marchetti, C., Romano, M., and Baralle, F. E. (2005) J. Mol. Biol. 348 575-588 - PubMed
-
- Wang, H. Y., Wang, I. F., Bose, J., and Shen, C. K. (2004) Genomics 83 130-139 - PubMed
-
- Davidson, Y., Kelley, T., Mackenzie, I. R., Pickering-Brown, S., Du Plessis, D., Neary, D., Snowden, J. S., and Mann, D. M. (2007) Acta Neuropathol. 113 521-533 - PubMed
-
- Neumann, M., Sampathu, D. M., Kwong, L. K., Truax, A. C., Micsenyi, M. C., Chou, T. T., Bruce, J., Schuck, T., Grossman, M., Clark, C. M., McCluskey, L. F., Miller, B. L., Masliah, E., Mackenzie, I. R., Feldman, H., Feiden, W., Kretzschmar, H. A., Trojanowski, J. Q., and Lee, V. M.-Y. (2006) Science 314 130-133 - PubMed
-
- Arai, T., Hasegawa, M., Akiyama, H., Ikeda, K., Nonaka, T., Mori, H., Mann, D., Tsuchiya, K., Yoshida, M., Hashizume, Y., and Oda, T. (2006) Biochem. Biophys. Res. Commun. 351 602-611 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

