Identification and characterization of the product encoded by ORF69 of Kaposi's sarcoma-associated herpesvirus
- PMID: 18305046
- PMCID: PMC2293072
- DOI: 10.1128/JVI.02400-07
Identification and characterization of the product encoded by ORF69 of Kaposi's sarcoma-associated herpesvirus
Abstract
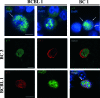
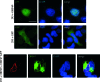
We report the identification and characterization of p33, the product of Kaposi's sarcoma-associated herpesvirus (KSHV) open reading frame 69 (ORF69), a positional homolog of the conserved herpesvirus protein UL31. p33 is expressed upon induction of viral lytic cycle with early kinetics. Immunofluorescence analysis revealed that in infected cell lines, the protein is localized in the nucleus, both in dotted spots and along the nuclear membrane. Nuclear fractionation experiments showed that p33 partitions with the nuclear matrix, and both immunoblotting of purified virions and immunoelectron microscopy indicated that the novel protein is not a component of the mature virus. Following ectopic expression in KSHV-negative cells, the protein was never associated with the nuclear membrane, suggesting that p33 needs to interact with additional viral proteins to reach the nuclear rim. In fact, after cotransfection with the ORF67 gene, the KSHV positional homolog of UL34, the p33 intranuclear signal changed and the two proteins colocalized on the nuclear membrane. A similar result was obtained when ORF69 was cotransfected with BFRF1, the Epstein-Barr virus (EBV) positional homolog of UL34 and ORF67. Finally, upon cotransfection, ORF69 significantly increased nuclear membrane reduplications induced by BFRF1. The above results indicate that KSHV p33 shares many similarities with its EBV homolog BFLF2 and suggest that functional cross-complementation is possible between members of the gammaherpesvirus subfamily.
Figures

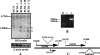
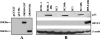



Similar articles
-
Characterization and intracellular localization of the Epstein-Barr virus protein BFLF2: interactions with BFRF1 and with the nuclear lamina.J Virol. 2005 Mar;79(6):3713-27. doi: 10.1128/JVI.79.6.3713-3727.2005. J Virol. 2005. PMID: 15731265 Free PMC article.
-
Interactions of the Kaposi's Sarcoma-associated herpesvirus nuclear egress complex: ORF69 is a potent factor for remodeling cellular membranes.J Virol. 2013 Apr;87(7):3915-29. doi: 10.1128/JVI.03418-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365436 Free PMC article.
-
Functional Identification and Characterization of the Nuclear Egress Complex of a Gammaherpesvirus.J Virol. 2019 Nov 26;93(24):e01422-19. doi: 10.1128/JVI.01422-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31554685 Free PMC article.
-
KSHV ORF67 encoded lytic protein localizes on the nuclear membrane and alters emerin distribution.Virus Res. 2013 Aug;175(2):143-50. doi: 10.1016/j.virusres.2013.04.001. Epub 2013 Apr 23. Virus Res. 2013. PMID: 23623980
-
[Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV, HHV-8)].Uirusu. 2010 Dec;60(2):237-45. doi: 10.2222/jsv.60.237. Uirusu. 2010. PMID: 21488336 Review. Japanese.
Cited by
-
Herpesvirus interactions with the host cytoskeleton.J Virol. 2009 Mar;83(5):2058-66. doi: 10.1128/JVI.01718-08. Epub 2008 Oct 8. J Virol. 2009. PMID: 18842724 Free PMC article. Review. No abstract available.
-
Herpes simplex virus 1 pUL34 plays a critical role in cell-to-cell spread of virus in addition to its role in virus replication.J Virol. 2011 Jul;85(14):7203-15. doi: 10.1128/JVI.00262-11. Epub 2011 May 11. J Virol. 2011. PMID: 21561917 Free PMC article.
-
KSHV reduces autophagy in THP-1 cells and in differentiating monocytes by decreasing CAST/calpastatin and ATG5 expression.Autophagy. 2016 Dec;12(12):2311-2325. doi: 10.1080/15548627.2016.1235122. Epub 2016 Oct 7. Autophagy. 2016. PMID: 27715410 Free PMC article.
-
Comprehensive mapping and analysis of Kaposi's sarcoma-associated herpesvirus 3' UTRs identify differential posttranscriptional control of gene expression in lytic versus latent infection.J Virol. 2013 Dec;87(23):12838-49. doi: 10.1128/JVI.02374-13. Epub 2013 Sep 25. J Virol. 2013. PMID: 24067953 Free PMC article.
-
Cell Culture Evolution of a Herpes Simplex Virus 1 (HSV-1)/Varicella-Zoster Virus (VZV) UL34/ORF24 Chimeric Virus Reveals Novel Functions for HSV Genes in Capsid Nuclear Egress.J Virol. 2021 Nov 9;95(23):e0095721. doi: 10.1128/JVI.00957-21. Epub 2021 Sep 15. J Virol. 2021. PMID: 34523964 Free PMC article.
References
-
- Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwich, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75). Int. J. Cancer 1927-33. - PubMed
-
- Ben-Yehoyada, M., I. Ben-Dor, and Y. Shaul. 2003. c-Abl tyrosine kinase selectively regulates p73 nuclear matrix association. J. Biol. Chem. 27834475-34482. - PubMed
-
- Boshoff, C., and R. Weiss. 2002. AIDS-related malignancies. Nat. Rev. Cancer 2373-382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

