Stem cells and niches: mechanisms that promote stem cell maintenance throughout life
- PMID: 18295578
- PMCID: PMC4505728
- DOI: 10.1016/j.cell.2008.01.038
Stem cells and niches: mechanisms that promote stem cell maintenance throughout life
Abstract
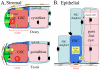
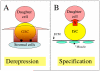
Niches are local tissue microenvironments that maintain and regulate stem cells. Long-predicted from mammalian studies, these structures have recently been characterized within several invertebrate tissues using methods that reliably identify individual stem cells and their functional requirements. Although similar single-cell resolution has usually not been achieved in mammalian tissues, principles likely to govern the behavior of niches in diverse organisms are emerging. Considerable progress has been made in elucidating how the microenvironment promotes stem cell maintenance. Mechanisms of stem cell maintenance are key to the regulation of homeostasis and likely contribute to aging and tumorigenesis when altered during adulthood.
Figures

Similar articles
-
Stem Cell Microenvironments and Beyond.Adv Exp Med Biol. 2017;1041:1-3. doi: 10.1007/978-3-319-69194-7_1. Adv Exp Med Biol. 2017. PMID: 29204825
-
Concise review: The plasticity of stem cell niches: a general property behind tissue homeostasis and repair.Stem Cells. 2014 Apr;32(4):852-9. doi: 10.1002/stem.1621. Stem Cells. 2014. PMID: 24356972 Review.
-
Breaking out of the mold: diversity within adult stem cells and their niches.Curr Opin Genet Dev. 2006 Oct;16(5):463-8. doi: 10.1016/j.gde.2006.08.003. Epub 2006 Aug 17. Curr Opin Genet Dev. 2006. PMID: 16919446 Review.
-
Finding a niche: studies from the Drosophila ovary.Stem Cell Res Ther. 2011 Nov 25;2(6):45. doi: 10.1186/scrt86. Stem Cell Res Ther. 2011. PMID: 22117545 Free PMC article. Review.
-
Stem cells and the niche: a dynamic duo.Cell Stem Cell. 2010 Feb 5;6(2):103-15. doi: 10.1016/j.stem.2010.01.011. Cell Stem Cell. 2010. PMID: 20144784 Free PMC article. Review.
Cited by
-
The miR-310/13 cluster antagonizes β-catenin function in the regulation of germ and somatic cell differentiation in the Drosophila testis.Development. 2013 Jul;140(14):2904-16. doi: 10.1242/dev.092817. Development. 2013. PMID: 23821034 Free PMC article.
-
Alternative splicing switching in stem cell lineages.Front Biol (Beijing). 2013 Feb 1;8(1):50-59. doi: 10.1007/s11515-012-1198-y. Front Biol (Beijing). 2013. PMID: 23399987 Free PMC article.
-
Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells.Cell. 2015 May 21;161(5):1175-1186. doi: 10.1016/j.cell.2015.04.001. Cell. 2015. PMID: 26000486 Free PMC article.
-
Autocrine platelet-derived growth factor-vascular endothelial growth factor receptor-related (Pvr) pathway activity controls intestinal stem cell proliferation in the adult Drosophila midgut.J Biol Chem. 2012 Aug 10;287(33):27359-70. doi: 10.1074/jbc.M112.378018. Epub 2012 Jun 21. J Biol Chem. 2012. PMID: 22722927 Free PMC article.
-
Characterization of spontaneous bone marrow recovery after sublethal total body irradiation: importance of the osteoblastic/adipocytic balance.PLoS One. 2012;7(2):e30818. doi: 10.1371/journal.pone.0030818. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363493 Free PMC article.
References
-
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. - PubMed
-
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nature Immunology. 2006;7:333–337. - PubMed
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

