Unfolded protein response and cell death after depletion of brefeldin A-inhibited guanine nucleotide-exchange protein GBF1
- PMID: 18287014
- PMCID: PMC2268553
- DOI: 10.1073/pnas.0712224105
Unfolded protein response and cell death after depletion of brefeldin A-inhibited guanine nucleotide-exchange protein GBF1
Abstract
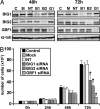
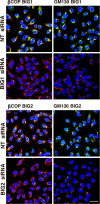
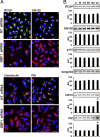
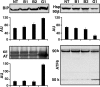
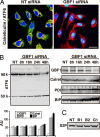
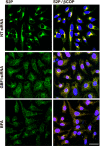
Guanine nucleotide-exchange factors (GEFs) activate ADP-ribosylation factor (ARF) GTPases that recruit coat proteins to membranes to initiate transport vesicle formation. Three mammalian GEFs are inhibited by brefeldin A (BFA). GBF1, predominantly associated with cis-Golgi membranes, functions early in the secretory pathway, whereas BIG1 and BIG2 act in trans-Golgi or later sites. Perturbation of endoplasmic reticulum (ER) functions can result in accumulation of unfolded or misfolded proteins that causes ER stress and unfolded protein response (UPR), with accumulation of ER stress response element (ERSE) gene products. BFA treatment of cells causes accumulation of proteins in the ER, ER stress, and ultimately apoptosis. To assess involvement of BFA-sensitive GEFs in the damage resulting from prolonged BFA treatment, HepG2 cells were selectively depleted of BIG1, BIG2, or GBF1 by using specific siRNA. Only GBF1 siRNA dramatically slowed cell growth, led to cell-cycle arrest in G(0)/G(1) phase, and caused dispersion of Golgi markers beta-COP and GM130, whereas ER structure appeared intact. GBF1 depletion also significantly increased levels of ER proteins calreticulin and protein disulfide isomerase (PDI). Proteomic analysis identified ER chaperones involved in the UPR that were significantly increased in amounts in GBF1-depleted cells. Upon ER stress, transcription factor ATF6 translocates from the ER to Golgi, where it is sequentially cleaved by site 1 and site 2 proteases, S1P and S2P, to a 50-kDa form that activates transcription of ERSE genes. Depletion of GBF1, but not BIG1 or BIG2, induced relocation of S2P from Golgi to ER with proteolysis of ATF6 followed by up-regulation of ER chaperones, mimicking a UPR response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
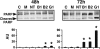
Similar articles
-
Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic.Mol Biol Cell. 2002 Jan;13(1):119-33. doi: 10.1091/mbc.01-08-0420. Mol Biol Cell. 2002. PMID: 11809827 Free PMC article.
-
Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi.Mol Biol Cell. 2005 Mar;16(3):1213-22. doi: 10.1091/mbc.e04-07-0599. Epub 2004 Dec 22. Mol Biol Cell. 2005. PMID: 15616190 Free PMC article.
-
Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking.J Cell Sci. 2007 Nov 15;120(Pt 22):3929-40. doi: 10.1242/jcs.010769. Epub 2007 Oct 23. J Cell Sci. 2007. PMID: 17956946
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
-
Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses.Viruses. 2020 Jun 24;12(6):682. doi: 10.3390/v12060682. Viruses. 2020. PMID: 32599855 Free PMC article. Review.
Cited by
-
Loss of soluble N-ethylmaleimide-sensitive factor attachment protein α (αSNAP) induces epithelial cell apoptosis via down-regulation of Bcl-2 expression and disruption of the Golgi.J Biol Chem. 2012 Feb 17;287(8):5928-41. doi: 10.1074/jbc.M111.278358. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194596 Free PMC article.
-
Protein folding stress potentiates NLRP1 and CARD8 inflammasome activation.Cell Rep. 2023 Jan 31;42(1):111965. doi: 10.1016/j.celrep.2022.111965. Epub 2023 Jan 16. Cell Rep. 2023. PMID: 36649711 Free PMC article.
-
Elucidation of toxicity pathways in lung epithelial cells induced by silicon dioxide nanoparticles.PLoS One. 2013 Sep 4;8(9):e72363. doi: 10.1371/journal.pone.0072363. eCollection 2013. PLoS One. 2013. PMID: 24023737 Free PMC article.
-
The small GTPase Arf1 modulates mitochondrial morphology and function.EMBO J. 2014 Nov 18;33(22):2659-75. doi: 10.15252/embj.201489039. Epub 2014 Sep 4. EMBO J. 2014. PMID: 25190516 Free PMC article.
-
COPI Vesicle Disruption Inhibits Mineralization via mTORC1-Mediated Autophagy.Int J Mol Sci. 2023 Dec 26;25(1):339. doi: 10.3390/ijms25010339. Int J Mol Sci. 2023. PMID: 38203512 Free PMC article.
References
-
- Moss J, Vaughan M. Molecules in the ARF orbit. J Biol Chem. 1998;273:21431–21434. - PubMed
-
- Jackson CL, Casanova JE. Turning on ARF: The Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 2000;10:60–67. - PubMed
-
- Donaldson JD, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. - PubMed
-
- Randazzo PA, Yang YC, Rulka C, Kahn RA. Activation of ADP-ribosylation factor by Golgi membranes: Evidence for a brefeldin A- and protease-sensitive activating factor on Golgi membranes. J Biol Chem. 1993;268:9555–9563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

