IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy
- PMID: 18276844
- PMCID: PMC2396726
- DOI: 10.1182/blood-2007-09-113050
IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy
Abstract
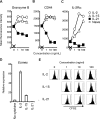
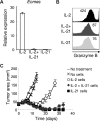
IL-2 and IL-21 are closely related cytokines that might have arisen by gene duplication. Both cytokines promote the function of effector CD8(+) T cells, but their distinct effects on antigen-driven differentiation of naive CD8(+) T cells into effector CD8(+) T cells are not clearly understood. We found that antigen-induced expression of Eomesodermin (Eomes) and maturation of naive CD8(+) T cells into granzyme B- and CD44-expressing effector CD8(+) T cells was enhanced by IL-2, but, unexpectedly, suppressed by IL-21. Furthermore, IL-21 repressed expression of IL-2Ra and inhibited IL-2-mediated acquisition of a cytolytic CD8(+) T-cell phenotype. Despite its inhibitory effects, IL-21 did not induce anergy, but instead potently enhanced the capacity of cells to mediate tumor regression upon adoptive transfer. In contrast, IL-2 impaired the subsequent antitumor function of transferred cells. Gene expression studies revealed a distinct IL-21 program that was characterized phenotypically by increased expression of L-selectin and functionally by enhanced antitumor immunity that was not reversed by secondary in vitro stimulation with antigen and IL-2. Thus, the efficacy of CD8(+) T cells for adoptive immunotherapy can be influenced by opposing differentiation programs conferred by IL-2 and IL-21, a finding with important implications for the development of cellular cancer therapies.
Figures
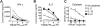
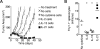

Comment in
-
IL-21 priming enhances T-cell immunotherapy.Blood. 2008 Jun 1;111(11):5268-9. doi: 10.1182/blood-2008-03-142505. Blood. 2008. PMID: 18502841 No abstract available.
Similar articles
-
Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGF-β1 signaling.J Immunol. 2013 Oct 1;191(7):3712-24. doi: 10.4049/jimmunol.1300319. Epub 2013 Sep 4. J Immunol. 2013. PMID: 24006458
-
Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells.Immunity. 2010 Jan 29;32(1):79-90. doi: 10.1016/j.immuni.2009.11.012. Epub 2010 Jan 21. Immunity. 2010. PMID: 20096607 Free PMC article.
-
A Context-Dependent Role for IL-21 in Modulating the Differentiation, Distribution, and Abundance of Effector and Memory CD8 T Cell Subsets.J Immunol. 2016 Mar 1;196(5):2153-66. doi: 10.4049/jimmunol.1401236. Epub 2016 Jan 29. J Immunol. 2016. PMID: 26826252 Free PMC article.
-
Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy.Immunity. 2013 Jan 24;38(1):13-25. doi: 10.1016/j.immuni.2013.01.004. Immunity. 2013. PMID: 23352221 Free PMC article. Review.
-
Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy.Immunol Rev. 2014 Jan;257(1):264-276. doi: 10.1111/imr.12135. Immunol Rev. 2014. PMID: 24329803 Free PMC article. Review.
Cited by
-
Ex vivo expansion of human T cells for adoptive immunotherapy using the novel Xeno-free CTS Immune Cell Serum Replacement.Clin Transl Immunology. 2015 Jan 16;4(1):e31. doi: 10.1038/cti.2014.31. eCollection 2015 Jan. Clin Transl Immunology. 2015. PMID: 25671129 Free PMC article.
-
Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype.Proc Natl Acad Sci U S A. 2012 Mar 20;109(12):4592-7. doi: 10.1073/pnas.1113748109. Epub 2012 Mar 5. Proc Natl Acad Sci U S A. 2012. PMID: 22393002 Free PMC article.
-
The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells.Immunity. 2013 Mar 21;38(3):514-27. doi: 10.1016/j.immuni.2013.02.011. Epub 2013 Feb 28. Immunity. 2013. PMID: 23453633 Free PMC article.
-
Stat5 opposes the transcription factor Tox and rewires exhausted CD8+ T cells toward durable effector-like states during chronic antigen exposure.Immunity. 2023 Dec 12;56(12):2699-2718.e11. doi: 10.1016/j.immuni.2023.11.005. Immunity. 2023. PMID: 38091951 Free PMC article.
-
CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies.Front Immunol. 2022 Sep 28;13:1019115. doi: 10.3389/fimmu.2022.1019115. eCollection 2022. Front Immunol. 2022. PMID: 36248810 Free PMC article. Review.
References
-
- Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. - PubMed
-
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

