Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits
- PMID: 18273889
- PMCID: PMC4947410
- DOI: 10.1002/cne.21665
Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits
Abstract
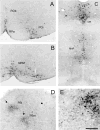
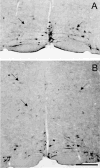
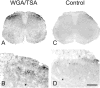
Despite the evidence for a significant contribution of brainstem serotonergic (5HT) systems to the control of spinal cord "pain" transmission neurons, attention has turned recently to the influence of nonserotonergic neurons, including the facilitatory and inhibitory controls that originate from so-called "on" and "off" cells of the rostroventral medulla (RVM). Unclear, however, is the extent to which these latter circuits interact with or are influenced by the serotonergic cell groups. To address this question we selectively targeted expression of a transneuronal tracer, wheat germ agglutinin (WGA), in the 5HT neurons so as to study the interplay between the 5HT and non-5HT systems. In addition to confirming the direct medullary 5HT projection to the spinal cord we also observed large numbers of non-5HT neurons, in the medullary nucleus reticularis gigantocellularis and magnocellularis, that were WGA-immunoreactive, i.e., were transneuronally labeled from 5HT neurons. FluoroGold injections into the spinal cord established that these reticular neurons are not only postsynaptic to the 5HT neurons of the medulla, but that most are also at the origin of descending, bulbospinal pathways. By contrast, we found no evidence that neurons of the midbrain periaqueductal gray that project to the RVM are postsynaptic to midbrain or medullary 5HT neurons. Finally, we found very few examples of WGA-immunoreactive noradrenergic neurons, which suggests that there is considerable independence of the monoaminergic bulbospinal pathways. Our results indicate that 5HT neurons influence "pain" processing at the spinal cord level both directly and indirectly via feedforward connections with multiple non-5HT descending control pathways.
(c) 2008 Wiley-Liss, Inc.
Figures






Similar articles
-
Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing.J Comp Neurol. 2009 May 10;514(2):145-60. doi: 10.1002/cne.22003. J Comp Neurol. 2009. PMID: 19274668 Free PMC article.
-
GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization.J Comp Neurol. 2004 Nov 15;479(3):257-70. doi: 10.1002/cne.20332. J Comp Neurol. 2004. PMID: 15457502
-
GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat.J Comp Neurol. 1991 Nov 8;313(2):349-67. doi: 10.1002/cne.903130210. J Comp Neurol. 1991. PMID: 1722490
-
Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat.Neuroscience. 1994 Oct;62(4):1155-78. doi: 10.1016/0306-4522(94)90351-4. Neuroscience. 1994. PMID: 7845592 Review.
-
Descending control of nociception: Specificity, recruitment and plasticity.Brain Res Rev. 2009 Apr;60(1):214-25. doi: 10.1016/j.brainresrev.2008.12.009. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19146877 Free PMC article. Review.
Cited by
-
Postnatal maturation of the spinal-bulbo-spinal loop: brainstem control of spinal nociception is independent of sensory input in neonatal rats.Pain. 2016 Mar;157(3):677-686. doi: 10.1097/j.pain.0000000000000420. Pain. 2016. PMID: 26574823 Free PMC article.
-
Gene transfer in the nervous system and implications for transsynaptic neuronal tracing.Expert Opin Biol Ther. 2010 May;10(5):763-72. doi: 10.1517/14712591003796538. Expert Opin Biol Ther. 2010. PMID: 20367126 Free PMC article. Review.
-
Peripheral and central neuronal ATF3 precedes CD4+ T-cell infiltration in EAE.Exp Neurol. 2016 Sep;283(Pt A):224-34. doi: 10.1016/j.expneurol.2016.06.019. Epub 2016 Jun 23. Exp Neurol. 2016. PMID: 27343802 Free PMC article.
-
Inhibition of itch-related responses by selectively ablated serotonergic signals at the rostral ventromedial medulla in mice.Int J Clin Exp Pathol. 2014 Dec 1;7(12):8917-21. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25674265 Free PMC article.
-
Inflammatory pain and corticosterone response in infant rats: effect of 5-HT1A agonist buspirone prior to gestational stress.Mediators Inflamm. 2013;2013:915189. doi: 10.1155/2013/915189. Epub 2013 Mar 31. Mediators Inflamm. 2013. PMID: 23606797 Free PMC article.
References
-
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain–medullary interactions in the modulation of pain. J Comp Neurol. 1981;201:285–297. - PubMed
-
- Akil H, Mayer DJ. Antagonism of stimulation-produced analgesia by p-CPA, a serotonin synthesis inhibitor. Brain Res. 1972;44:692–697. - PubMed
-
- Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res. 1985;343:223–229. - PubMed
-
- Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–531. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

