Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women
- PMID: 18270595
- PMCID: PMC2229663
- DOI: 10.1371/journal.pone.0001608
Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women
Abstract
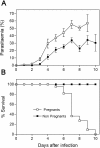
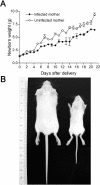
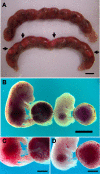
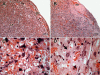
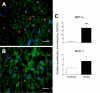
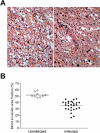
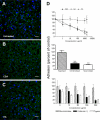
Pregnancy-associated malaria (PAM) is expressed in a range of clinical complications that include increased disease severity in pregnant women, decreased fetal viability, intra-uterine growth retardation, low birth weight and infant mortality. The physiopathology of malaria in pregnancy is difficult to scrutinize and attempts were made in the past to use animal models for pregnancy malaria studies. Here, we describe a comprehensive mouse experimental model that recapitulates many of the pathological and clinical features typical of human severe malaria in pregnancy. We used P. berghei ANKA-GFP infection during pregnancy to evoke a prominent inflammatory response in the placenta that entails CD11b mononuclear infiltration, up-regulation of MIP-1 alpha chemokine and is associated with marked reduction of placental vascular spaces. Placenta pathology was associated with decreased fetal viability, intra-uterine growth retardation, gross post-natal growth impairment and increased disease severity in pregnant females. Moreover, we provide evidence that CSA and HA, known to mediate P. falciparum adhesion to human placenta, are also involved in mouse placental malaria infection. We propose that reduction of maternal blood flow in the placenta is a key pathogenic factor in murine pregnancy malaria and we hypothesize that exacerbated innate inflammatory responses to Plasmodium infected red blood cells trigger severe placenta pathology. This experimental model provides an opportunity to identify cell and molecular components of severe PAM pathogenesis and to investigate the inflammatory response that leads to the observed fetal and placental blood circulation abnormalities.
Conflict of interest statement
Figures

Similar articles
-
Murine Model for Preclinical Studies of Var2CSA-Mediated Pathology Associated with Malaria in Pregnancy.Infect Immun. 2016 May 24;84(6):1761-1774. doi: 10.1128/IAI.01207-15. Print 2016 Jun. Infect Immun. 2016. PMID: 27045035 Free PMC article.
-
Distinct placental malaria pathology caused by different Plasmodium berghei lines that fail to induce cerebral malaria in the C57BL/6 mouse.Malar J. 2012 Jul 16;11:231. doi: 10.1186/1475-2875-11-231. Malar J. 2012. PMID: 22799533 Free PMC article.
-
Maternal and fetal outcome of pregnancy in Swiss mice infected with Plasmodium berghei ANKAGFP.Reprod Toxicol. 2019 Oct;89:107-114. doi: 10.1016/j.reprotox.2019.07.011. Epub 2019 Jul 13. Reprod Toxicol. 2019. PMID: 31310803
-
Of mice and women: rodent models of placental malaria.Trends Parasitol. 2010 Aug;26(8):412-9. doi: 10.1016/j.pt.2010.04.010. Epub 2010 May 31. Trends Parasitol. 2010. PMID: 20605743 Review.
-
The placenta and malaria.Ann Trop Med Parasitol. 1997 Oct;91(7):803-10. doi: 10.1080/00034989760563. Ann Trop Med Parasitol. 1997. PMID: 9625937 Review.
Cited by
-
Murine Model for Preclinical Studies of Var2CSA-Mediated Pathology Associated with Malaria in Pregnancy.Infect Immun. 2016 May 24;84(6):1761-1774. doi: 10.1128/IAI.01207-15. Print 2016 Jun. Infect Immun. 2016. PMID: 27045035 Free PMC article.
-
Differential roles of inflammation and apoptosis in initiation of mid-gestational abortion in malaria-infected C57BL/6 and A/J mice.Placenta. 2015 Jul;36(7):738-49. doi: 10.1016/j.placenta.2015.04.007. Epub 2015 Apr 28. Placenta. 2015. PMID: 25956987 Free PMC article.
-
Effect of mushroom Agaricus blazei on immune response and development of experimental cerebral malaria.Malar J. 2015 Aug 11;14:311. doi: 10.1186/s12936-015-0832-y. Malar J. 2015. PMID: 26260055 Free PMC article.
-
Distinct placental malaria pathology caused by different Plasmodium berghei lines that fail to induce cerebral malaria in the C57BL/6 mouse.Malar J. 2012 Jul 16;11:231. doi: 10.1186/1475-2875-11-231. Malar J. 2012. PMID: 22799533 Free PMC article.
-
Contribution of Murine Models to the Study of Malaria During Pregnancy.Front Microbiol. 2019 Jun 19;10:1369. doi: 10.3389/fmicb.2019.01369. eCollection 2019. Front Microbiol. 2019. PMID: 31275284 Free PMC article. Review.
References
-
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. - PubMed
-
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. - PubMed
-
- Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–183. - PubMed
-
- Nosten F, Rogerson SJ, Beeson JG, McGready R, Mutabingwa TK, et al. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 2004;20:425–432. - PubMed
-
- Shulman CE, Graham WJ, Jilo H, Lowe BS, New L, et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1996;90:535–539. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

