Lovastatin inhibits amyloid precursor protein (APP) beta-cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts
- PMID: 18266936
- PMCID: PMC2707757
- DOI: 10.1111/j.1471-4159.2008.05283.x
Lovastatin inhibits amyloid precursor protein (APP) beta-cleavage through reduction of APP distribution in Lubrol WX extractable low density lipid rafts
Abstract
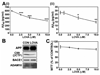
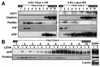
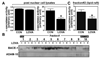
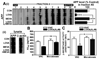
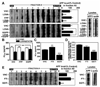
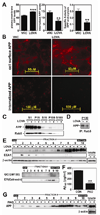
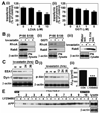
Previous studies have described that statins (inhibitors of cholesterol and isoprenoid biosynthesis) inhibit the output of amyloid-beta (Abeta) in the animal model and thus decrease risk of Alzheimer's disease. However, their action mechanism(s) in Abeta precursor protein (APP) processing and Abeta generation is not fully understood. In this study, we report that lovastatin treatment reduced Abeta output in cultured hippocampal neurons as a result of reduced APP levels and beta-secretase activities in low density Lubrol WX (non-ionic detergent) extractable lipid rafts (LDLR). Rather than altering cholesterol levels in lipid raft fractions and thus disrupting lipid raft structure, lovastatin decreased Abeta generation through down-regulating geranylgeranyl-pyrophosphate dependent endocytosis pathway. The inhibition of APP endocytosis by treatment with lovastatin and reduction of APP levels in LDLR fractions by treatment with phenylarsine oxide (a general endocytosis inhibitor) support the involvement of APP endocytosis in APP distribution in LDLR fractions and subsequent APP beta-cleavage. Moreover, lovastatin-mediated down-regulation of endocytosis regulators, such as early endosomal antigen 1, dynamin-1, and phosphatidylinositol 3-kinase activity, indicates that lovastatin modulates APP endocytosis possibly through its pleiotropic effects on endocytic regulators. Collectively, these data report that lovastatin mediates inhibition of LDLR distribution and beta-cleavage of APP in a geranylgeranyl-pyrophosphate and endocytosis-dependent manner.
Figures

Similar articles
-
Preferred Endocytosis of Amyloid Precursor Protein from Cholesterol-Enriched Lipid Raft Microdomains.Molecules. 2020 Nov 24;25(23):5490. doi: 10.3390/molecules25235490. Molecules. 2020. PMID: 33255194 Free PMC article.
-
Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma.FASEB J. 2008 Jan;22(1):47-54. doi: 10.1096/fj.07-8175com. Epub 2007 Jul 31. FASEB J. 2008. PMID: 17666454 Free PMC article.
-
Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts.J Cell Biol. 2003 Jan 6;160(1):113-23. doi: 10.1083/jcb.200207113. Epub 2003 Jan 6. J Cell Biol. 2003. PMID: 12515826 Free PMC article.
-
Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein.Biochem Soc Trans. 2005 Apr;33(Pt 2):335-8. doi: 10.1042/BST0330335. Biochem Soc Trans. 2005. PMID: 15787600 Review.
-
Ectodomain shedding of the amyloid precursor protein: cellular control mechanisms and novel modifiers.Neurodegener Dis. 2006;3(4-5):262-9. doi: 10.1159/000095265. Neurodegener Dis. 2006. PMID: 17047366 Review.
Cited by
-
Statins reduce amyloid β-peptide production by modulating amyloid precursor protein maturation and phosphorylation through a cholesterol-independent mechanism in cultured neurons.Neurochem Res. 2013 Mar;38(3):589-600. doi: 10.1007/s11064-012-0956-1. Epub 2012 Dec 28. Neurochem Res. 2013. PMID: 23269484
-
Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis.Biochem Biophys Res Commun. 2010 Sep 3;399(4):487-91. doi: 10.1016/j.bbrc.2010.07.081. Epub 2010 Jul 24. Biochem Biophys Res Commun. 2010. PMID: 20659426 Free PMC article.
-
Methodological Pitfalls of Investigating Lipid Rafts in the Brain: What Are We Still Missing?Biomolecules. 2024 Jan 28;14(2):156. doi: 10.3390/biom14020156. Biomolecules. 2024. PMID: 38397393 Free PMC article. Review.
-
Membrane rafts in Alzheimer's disease beta-amyloid production.Biochim Biophys Acta. 2010 Aug;1801(8):860-7. doi: 10.1016/j.bbalip.2010.03.007. Epub 2010 Mar 18. Biochim Biophys Acta. 2010. PMID: 20303415 Free PMC article. Review.
-
Surface expression and limited proteolysis of ADAM10 are increased by a dominant negative inhibitor of dynamin.BMC Cell Biol. 2011 May 17;12:20. doi: 10.1186/1471-2121-12-20. BMC Cell Biol. 2011. PMID: 21586144 Free PMC article.
References
-
- Andersson M, Elmberger PG, Edlund C, Kristensson K, Dallner G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990;269:15–18. - PubMed
-
- Antonny B. SNARE filtering by dynamin. Cell. 2004;119:581–582. - PubMed
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS022576/NS/NINDS NIH HHS/United States
- R01 NS034741/NS/NINDS NIH HHS/United States
- R37 NS022576/NS/NINDS NIH HHS/United States
- NS-22576/NS/NINDS NIH HHS/United States
- C06 RR018823/RR/NCRR NIH HHS/United States
- R56 AG025307-02/AG/NIA NIH HHS/United States
- RR018823/RR/NCRR NIH HHS/United States
- AG-25307/AG/NIA NIH HHS/United States
- NS-37766/NS/NINDS NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- R01 NS037766-10/NS/NINDS NIH HHS/United States
- R56 AG025307/AG/NIA NIH HHS/United States
- RR015455/RR/NCRR NIH HHS/United States
- R01 NS022576-17/NS/NINDS NIH HHS/United States
- R01 NS037766/NS/NINDS NIH HHS/United States
- R01 NS034741-12/NS/NINDS NIH HHS/United States
- NS-34741/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

