Acinus-S' represses retinoic acid receptor (RAR)-regulated gene expression through interaction with the B domains of RARs
- PMID: 18250153
- PMCID: PMC2293115
- DOI: 10.1128/MCB.01199-07
Acinus-S' represses retinoic acid receptor (RAR)-regulated gene expression through interaction with the B domains of RARs
Abstract
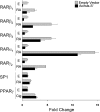
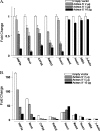
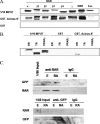
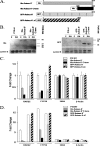
The diverse biological actions of retinoic acid (RA) are mediated by RA receptors (RARs) and retinoid X receptors (RXRs). Modulation of transcription by RARs/RXRs is achieved through two activation functions, ligand-independent AF-1 and ligand-dependent AF-2, located in the A/B and E domains, respectively. While the coregulatory proteins that interact with the E domain are well studied, the A/B domain-interacting partners and their influence(s) on the function of RARs are poorly understood. Acinus-S' is an ubiquitous nuclear protein that has been implicated in inducing apoptotic chromatin condensation and regulating mRNA processing. Our data demonstrate that Acinus-S' can specifically repress ligand-independent and ligand-dependent expression of a DR5 RA response element(RARE)-dependent reporter gene and several endogenous RAR-regulated genes in a dose-dependent and gene-specific manner. Chromatin immunoprecipitation assays show that Acinus-S' associates with RAREs within the promoters of endogenous genes independent of RA treatment. Furthermore, the C-terminal end of Acinus-S' and the B domain of RARbeta interact independently of ligand, and the C-terminal end of Acinus-S' is sufficient for the repression of RAR-regulated gene expression. Finally, histone deacetylase activity only partially accounts for the repressive effect of Acinus-S' on RAR-dependent gene expression. These findings identify Acinus-S' as a novel RAR-interacting protein that regulates the expression of a subset of RAR-regulated genes through direct binding to the N-terminal B domains of RARs.
Figures



Similar articles
-
Role of Acinus in regulating retinoic acid-responsive gene pre-mRNA splicing.J Cell Physiol. 2015 Apr;230(4):791-801. doi: 10.1002/jcp.24804. J Cell Physiol. 2015. PMID: 25205379 Free PMC article.
-
Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells.J Mol Biol. 2007 Sep 14;372(2):298-316. doi: 10.1016/j.jmb.2007.06.079. Epub 2007 Jul 3. J Mol Biol. 2007. PMID: 17663992 Free PMC article.
-
Distinct binding determinants for 9-cis retinoic acid are located within AF-2 of retinoic acid receptor alpha.Mol Cell Biol. 1994 Apr;14(4):2323-30. doi: 10.1128/mcb.14.4.2323-2330.1994. Mol Cell Biol. 1994. PMID: 8139538 Free PMC article.
-
[Genetic control of the development by retinoic acid].C R Seances Soc Biol Fil. 1997;191(1):77-90. C R Seances Soc Biol Fil. 1997. PMID: 9181129 Review. French.
-
Retinoids and receptor interacting protein 140 (RIP140) in gene regulation.Curr Med Chem. 2004 Jun;11(12):1527-32. doi: 10.2174/0929867043365017. Curr Med Chem. 2004. PMID: 15180561 Review.
Cited by
-
Human SAP18 mediates assembly of a splicing regulatory multiprotein complex via its ubiquitin-like fold.RNA. 2010 Dec;16(12):2442-54. doi: 10.1261/rna.2304410. Epub 2010 Oct 21. RNA. 2010. PMID: 20966198 Free PMC article.
-
The structure of the ASAP core complex reveals the existence of a Pinin-containing PSAP complex.Nat Struct Mol Biol. 2012 Mar 4;19(4):378-86. doi: 10.1038/nsmb.2242. Nat Struct Mol Biol. 2012. PMID: 22388736
-
Multifaceted Regulation of Gene Expression by the Apoptosis- and Splicing-Associated Protein Complex and Its Components.Int J Biol Sci. 2017 Apr 10;13(5):545-560. doi: 10.7150/ijbs.18649. eCollection 2017. Int J Biol Sci. 2017. PMID: 28539829 Free PMC article. Review.
-
NGF inhibits human leukemia proliferation by downregulating cyclin A1 expression through promoting acinus/CtBP2 association.Oncogene. 2009 Oct 29;28(43):3825-36. doi: 10.1038/onc.2009.236. Epub 2009 Aug 10. Oncogene. 2009. PMID: 19668232 Free PMC article.
-
The exon junction complex controls transposable element activity by ensuring faithful splicing of the piwi transcript.Genes Dev. 2014 Aug 15;28(16):1786-99. doi: 10.1101/gad.245829.114. Epub 2014 Aug 7. Genes Dev. 2014. PMID: 25104425 Free PMC article.
References
-
- Aravind, L., and E. V. Koonin. 2000. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25112-114. - PubMed
-
- Auboeuf, D., E. Batsche, M. Dutertre, C. Muchardt, and B. W. O'Malley. 2007. Coregulators: transducing signal from transcription to alternative splicing. Trends Endocrinol. Metab. 18122-129. - PubMed
-
- Bastien, J., and C. Rochette-Egly. 2004. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 3281-16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
