AsSIRTing the DNA damage response
- PMID: 18215521
- PMCID: PMC8483772
- DOI: 10.1016/j.tcb.2007.11.007
AsSIRTing the DNA damage response
Abstract
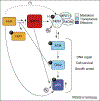
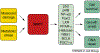
In mammalian cells, changes in signaling networks and expressed proteins ensure the adequate detection and management of damaged macromolecules. Here, we review an emergent pathway of maintenance of homeostasis following genotoxic stress. The RNA-binding protein HuR associates with sirtuin (SIRT)1 mRNA and maintains constitutively elevated levels of SIRT1 protein, a deacetylase that elicits a prosurvival function. SIRT1 was recently shown to deacetylate the Nijmegen breakage syndrome (NBS1) protein, thereby rendering it phosphorylatable by ataxia telangiectasia mutated protein (ATM). A component of the MRN (MRE11-RAD50-NBS1) nuclease complex, NBS1 is crucial for sensing DNA damage and mounting a genotoxic response. This article covers the regulatory pathway of HuR-->SIRT1-->NBS1, through which post-transcriptional and post-translational effectors contribute to the maintenance of genomic integrity.
Figures

Similar articles
-
SIRT1 regulates the function of the Nijmegen breakage syndrome protein.Mol Cell. 2007 Jul 6;27(1):149-62. doi: 10.1016/j.molcel.2007.05.029. Mol Cell. 2007. PMID: 17612497 Free PMC article.
-
Phosphorylation of HuR by Chk2 regulates SIRT1 expression.Mol Cell. 2007 Feb 23;25(4):543-57. doi: 10.1016/j.molcel.2007.01.011. Mol Cell. 2007. PMID: 17317627 Free PMC article.
-
Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review.
-
ATM regulates a DNA damage response posttranscriptional RNA operon in lymphocytes.Blood. 2011 Feb 24;117(8):2441-50. doi: 10.1182/blood-2010-09-310987. Epub 2011 Jan 5. Blood. 2011. PMID: 21209379 Free PMC article.
-
Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template.Biochem Cell Biol. 2007 Aug;85(4):509-20. doi: 10.1139/O07-069. Biochem Cell Biol. 2007. PMID: 17713585 Review.
Cited by
-
Angiogenesis inhibitor vasohibin-1 enhances stress resistance of endothelial cells via induction of SOD2 and SIRT1.PLoS One. 2012;7(10):e46459. doi: 10.1371/journal.pone.0046459. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056314 Free PMC article.
-
Why Human Papillomaviruses Activate the DNA Damage Response (DDR) and How Cellular and Viral Replication Persists in the Presence of DDR Signaling.Viruses. 2017 Sep 21;9(10):268. doi: 10.3390/v9100268. Viruses. 2017. PMID: 28934154 Free PMC article. Review.
-
SIRT1 as a therapeutic target in inflammaging of the pulmonary disease.Prev Med. 2012 May;54 Suppl(Suppl):S20-8. doi: 10.1016/j.ypmed.2011.11.014. Epub 2011 Dec 8. Prev Med. 2012. PMID: 22178470 Free PMC article. Review.
-
Synergistic Immunostimulatory Effects and Therapeutic Benefit of Combined Histone Deacetylase and Bromodomain Inhibition in Non-Small Cell Lung Cancer.Cancer Discov. 2017 Aug;7(8):852-867. doi: 10.1158/2159-8290.CD-16-1020. Epub 2017 Apr 13. Cancer Discov. 2017. PMID: 28408401 Free PMC article.
-
Epigenetic Targeting of Autophagy via HDAC Inhibition in Tumor Cells: Role of p53.Int J Mol Sci. 2018 Dec 8;19(12):3952. doi: 10.3390/ijms19123952. Int J Mol Sci. 2018. PMID: 30544838 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

