In silico detection of sequence variations modifying transcriptional regulation
- PMID: 18208319
- PMCID: PMC2211530
- DOI: 10.1371/journal.pcbi.0040005
In silico detection of sequence variations modifying transcriptional regulation
Abstract
Identification of functional genetic variation associated with increased susceptibility to complex diseases can elucidate genes and underlying biochemical mechanisms linked to disease onset and progression. For genes linked to genetic diseases, most identified causal mutations alter an encoded protein sequence. Technological advances for measuring RNA abundance suggest that a significant number of undiscovered causal mutations may alter the regulation of gene transcription. However, it remains a challenge to separate causal genetic variations from linked neutral variations. Here we present an in silico driven approach to identify possible genetic variation in regulatory sequences. The approach combines phylogenetic footprinting and transcription factor binding site prediction to identify variation in candidate cis-regulatory elements. The bioinformatics approach has been tested on a set of SNPs that are reported to have a regulatory function, as well as background SNPs. In the absence of additional information about an analyzed gene, the poor specificity of binding site prediction is prohibitive to its application. However, when additional data is available that can give guidance on which transcription factor is involved in the regulation of the gene, the in silico binding site prediction improves the selection of candidate regulatory polymorphisms for further analyses. The bioinformatics software generated for the analysis has been implemented as a Web-based application system entitled RAVEN (regulatory analysis of variation in enhancers). The RAVEN system is available at http://www.cisreg.ca for all researchers interested in the detection and characterization of regulatory sequence variation.
Conflict of interest statement
Figures
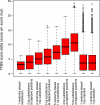
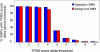
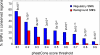
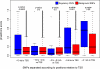


Similar articles
-
BLISS 2.0: a web-based tool for predicting conserved regulatory modules in distantly-related orthologous sequences.Bioinformatics. 2007 Dec 1;23(23):3249-50. doi: 10.1093/bioinformatics/btm368. Epub 2007 Jul 27. Bioinformatics. 2007. PMID: 17660203 Free PMC article.
-
A preliminary computational outputs versus experimental results: Application of sTRAP, a biophysical tool for the analysis of SNPs of transcription factor-binding sites.Mol Genet Genomic Med. 2020 May;8(5):e1219. doi: 10.1002/mgg3.1219. Epub 2020 Mar 10. Mol Genet Genomic Med. 2020. PMID: 32155318 Free PMC article.
-
Computational detection of cis -regulatory modules.Bioinformatics. 2003 Oct;19 Suppl 2:ii5-14. doi: 10.1093/bioinformatics/btg1052. Bioinformatics. 2003. PMID: 14534164
-
Single nucleotide polymorphism in transcriptional regulatory regions and expression of environmentally responsive genes.Toxicol Appl Pharmacol. 2005 Sep 1;207(2 Suppl):84-90. doi: 10.1016/j.taap.2004.09.024. Toxicol Appl Pharmacol. 2005. PMID: 16002116 Review.
-
Bioinformatics approaches and resources for single nucleotide polymorphism functional analysis.Brief Bioinform. 2005 Mar;6(1):44-56. doi: 10.1093/bib/6.1.44. Brief Bioinform. 2005. PMID: 15826356 Review.
Cited by
-
atSNP Search: a web resource for statistically evaluating influence of human genetic variation on transcription factor binding.Bioinformatics. 2019 Aug 1;35(15):2657-2659. doi: 10.1093/bioinformatics/bty1010. Bioinformatics. 2019. PMID: 30534948 Free PMC article.
-
Candidate SNP Markers of Chronopathologies Are Predicted by a Significant Change in the Affinity of TATA-Binding Protein for Human Gene Promoters.Biomed Res Int. 2016;2016:8642703. doi: 10.1155/2016/8642703. Epub 2016 Aug 22. Biomed Res Int. 2016. PMID: 27635400 Free PMC article.
-
A SNP in the HTT promoter alters NF-κB binding and is a bidirectional genetic modifier of Huntington disease.Nat Neurosci. 2015 Jun;18(6):807-16. doi: 10.1038/nn.4014. Epub 2015 May 4. Nat Neurosci. 2015. PMID: 25938884
-
CERENKOV2: improved detection of functional noncoding SNPs using data-space geometric features.BMC Bioinformatics. 2019 Feb 6;20(1):63. doi: 10.1186/s12859-019-2637-4. BMC Bioinformatics. 2019. PMID: 30727967 Free PMC article.
-
Prediction of LncRNA Subcellular Localization with Deep Learning from Sequence Features.Sci Rep. 2018 Nov 6;8(1):16385. doi: 10.1038/s41598-018-34708-w. Sci Rep. 2018. PMID: 30401954 Free PMC article.
References
-
- Kuriki C, Tanaka T, Fukui Y, Sato O, Motojima K. Structural and functional analysis of a new upstream promoter of the human FAT/CD36 gene. Biol Pharm Bull. 2002;25:1476–1478. - PubMed
-
- Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171–1175. - PubMed
-
- De Gobbi M, Viprakasit V, Hughes JR, Fisher C, Buckle VJ, et al. A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science. 2006;312:1215–1217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

