The viral oncoprotein LMP1 exploits TRADD for signaling by masking its apoptotic activity
- PMID: 18198944
- PMCID: PMC2174972
- DOI: 10.1371/journal.pbio.0060008
The viral oncoprotein LMP1 exploits TRADD for signaling by masking its apoptotic activity
Abstract
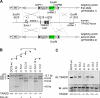
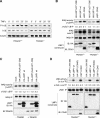
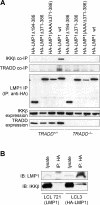
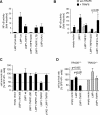
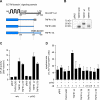
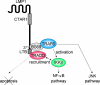
The tumor necrosis factor (TNF)-receptor 1-associated death domain protein (TRADD) mediates induction of apoptosis as well as activation of NF-kappaB by cellular TNF-receptor 1 (TNFR1). TRADD is also recruited by the latent membrane protein 1 (LMP1) oncoprotein of Epstein-Barr virus, but its role in LMP1 signaling has remained enigmatic. In human B lymphocytes, we have generated, to our knowledge, the first genetic knockout of TRADD to investigate TRADD's role in LMP1 signal transduction. Our data from TRADD-deficient cells demonstrate that TRADD is a critical signaling mediator of LMP1 that is required for LMP1 to recruit and activate I-kappaB kinase beta (IKKbeta). However, in contrast to TNFR1, LMP1-induced TRADD signaling does not induce apoptosis. Searching for the molecular basis for this observation, we characterized the 16 C-terminal amino acids of LMP1 as an autonomous and unique virus-derived TRADD-binding domain. Replacing the death domain of TNFR1 by LMP1's TRADD-binding domain converts TNFR1 into a nonapoptotic receptor that activates NF-kappaB through a TRAF6-dependent pathway, like LMP1 but unlike wild-type TNFR1. Thus, the unique interaction of LMP1 with TRADD encodes the transforming phenotype of viral TRADD signaling and masks TRADD's pro-apoptotic function.
Conflict of interest statement
Figures

Similar articles
-
Pursuing different 'TRADDes': TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1.Biol Chem. 2008 Oct;389(10):1261-71. doi: 10.1515/BC.2008.144. Biol Chem. 2008. PMID: 18713013 Review.
-
LMP1 signal transduction differs substantially from TNF receptor 1 signaling in the molecular functions of TRADD and TRAF2.EMBO J. 1999 May 4;18(9):2511-21. doi: 10.1093/emboj/18.9.2511. EMBO J. 1999. PMID: 10228165 Free PMC article.
-
The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-kappaB activation.Mol Cell Biol. 1999 Aug;19(8):5759-67. doi: 10.1128/MCB.19.8.5759. Mol Cell Biol. 1999. PMID: 10409763 Free PMC article.
-
Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2.J Virol. 1999 Feb;73(2):1023-35. doi: 10.1128/JVI.73.2.1023-1035.1999. J Virol. 1999. PMID: 9882303 Free PMC article.
-
The Latent Membrane Protein 1 (LMP1).Curr Top Microbiol Immunol. 2015;391:119-49. doi: 10.1007/978-3-319-22834-1_4. Curr Top Microbiol Immunol. 2015. PMID: 26428373 Review.
Cited by
-
A central role of IKK2 and TPL2 in JNK activation and viral B-cell transformation.Nat Commun. 2020 Feb 4;11(1):685. doi: 10.1038/s41467-020-14502-x. Nat Commun. 2020. PMID: 32019925 Free PMC article.
-
Viruses and breast cancer.Cancers (Basel). 2010 Apr 30;2(2):752-72. doi: 10.3390/cancers2020752. Cancers (Basel). 2010. PMID: 24281093 Free PMC article.
-
The tumor marker Fascin is induced by the Epstein-Barr virus-encoded oncoprotein LMP1 via NF-κB in lymphocytes and contributes to their invasive migration.Cell Commun Signal. 2014 Jul 11;12:46. doi: 10.1186/s12964-014-0046-x. Cell Commun Signal. 2014. PMID: 25105941 Free PMC article.
-
Epstein-Barr virus: the mastermind of immune chaos.Front Immunol. 2024 Feb 7;15:1297994. doi: 10.3389/fimmu.2024.1297994. eCollection 2024. Front Immunol. 2024. PMID: 38384471 Free PMC article. Review.
-
Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer.PLoS One. 2012;7(11):e48788. doi: 10.1371/journal.pone.0048788. Epub 2012 Nov 19. PLoS One. 2012. PMID: 23183846 Free PMC article.
References
-
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. - PubMed
-
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. - PubMed
-
- Kieser A. Signal transduction by the Epstein-Barr virus oncogene latent membrane protein 1 (LMP1) Signal Transduction. 2007;7:20–33.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

