The Drosophila cytosine-5 methyltransferase Dnmt2 is associated with the nuclear matrix and can access DNA during mitosis
- PMID: 18183295
- PMCID: PMC2169302
- DOI: 10.1371/journal.pone.0001414
The Drosophila cytosine-5 methyltransferase Dnmt2 is associated with the nuclear matrix and can access DNA during mitosis
Abstract
Cytosine-5 methyltransferases of the Dnmt2 family are highly conserved in evolution and their biological function is being studied in several organisms. Although all structural DNA methyltransferase motifs are present in Dnmt2, these enzymes show a strong tRNA methyltransferase activity. In line with an enzymatic activity towards substrates other than DNA, Dnmt2 has been described to localize to the cytoplasm. Using molecular and biochemical approaches we show here that Dnmt2 is both a cytoplasmic and a nuclear protein. Sub-cellular fractionation shows that a significant amount of Dnmt2 is bound to the nuclear matrix. Sub-cellular localization analysis reveals that Dnmt2 proteins are enriched in actively dividing cells. Dnmt2 localization is highly dynamic during the cell cycle. Using live imaging we observed that Dnmt2-EGFP enters prophase nuclei and shows a spindle-like localization pattern during mitotic divisions. Additional experiments suggest that this localization is microtubule dependent and that Dnmt2 can access DNA during mitotic cell divisions. Our results represent the first comprehensive characterization of Dnmt2 proteins on the cellular level and have important implications for our understanding of the molecular activities of Dnmt2.
Conflict of interest statement
Figures
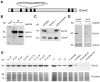
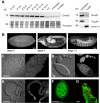

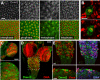
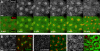

Similar articles
-
Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2.Science. 2006 Jan 20;311(5759):395-8. doi: 10.1126/science.1120976. Science. 2006. PMID: 16424344
-
A Dnmt2-like protein mediates DNA methylation in Drosophila.Development. 2003 Nov;130(21):5083-90. doi: 10.1242/dev.00716. Epub 2003 Aug 27. Development. 2003. PMID: 12944428
-
Two substrates are better than one: dual specificities for Dnmt2 methyltransferases.Trends Biochem Sci. 2006 Jun;31(6):306-8. doi: 10.1016/j.tibs.2006.04.005. Epub 2006 May 6. Trends Biochem Sci. 2006. PMID: 16679017 Review.
-
Conservation of DNA methylation in dipteran insects.Insect Mol Biol. 2004 Apr;13(2):117-23. doi: 10.1111/j.0962-1075.2004.00466.x. Insect Mol Biol. 2004. PMID: 15056358
-
[Dnmt2 is the Most Evolutionary Conserved and Enigmatic Cytosine DNA Methyltransferase in Eukaryotes].Genetika. 2016 Mar;52(3):269-82. Genetika. 2016. PMID: 27281847 Review. Russian.
Cited by
-
Mechanism and biological role of Dnmt2 in Nucleic Acid Methylation.RNA Biol. 2017 Sep 2;14(9):1108-1123. doi: 10.1080/15476286.2016.1191737. Epub 2016 May 27. RNA Biol. 2017. PMID: 27232191 Free PMC article. Review.
-
DNA methyltransferase 1 mutations and mitochondrial pathology: is mtDNA methylated?Front Genet. 2015 Mar 12;6:90. doi: 10.3389/fgene.2015.00090. eCollection 2015. Front Genet. 2015. PMID: 25815005 Free PMC article.
-
NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs.RNA. 2015 Sep;21(9):1532-43. doi: 10.1261/rna.051524.115. Epub 2015 Jul 9. RNA. 2015. PMID: 26160102 Free PMC article.
-
Molecular and enzymatic profiles of mammalian DNA methyltransferases: structures and targets for drugs.Curr Med Chem. 2010;17(33):4052-71. doi: 10.2174/092986710793205372. Curr Med Chem. 2010. PMID: 20939822 Free PMC article. Review.
-
Malaria Parasite Stress Tolerance Is Regulated by DNMT2-Mediated tRNA Cytosine Methylation.mBio. 2021 Dec 21;12(6):e0255821. doi: 10.1128/mBio.02558-21. Epub 2021 Nov 2. mBio. 2021. PMID: 34724812 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

