Domain requirement of moenomycin binding to bifunctional transglycosylases and development of high-throughput discovery of antibiotics
- PMID: 18182485
- PMCID: PMC2206553
- DOI: 10.1073/pnas.0710868105
Domain requirement of moenomycin binding to bifunctional transglycosylases and development of high-throughput discovery of antibiotics
Abstract
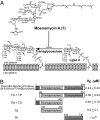
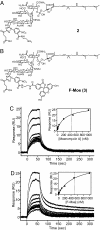
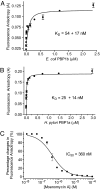
Moenomycin inhibits bacterial growth by blocking the transglycosylase activity of class A penicillin-binding proteins (PBPs), which are key enzymes in bacterial cell wall synthesis. We compared the binding affinities of moenomycin A with various truncated PBPs by using surface plasmon resonance analysis and found that the transmembrane domain is important for moenomycin binding. Full-length class A PBPs from 16 bacterial species were produced, and their binding activities showed a correlation with the antimicrobial activity of moenomycin against Enterococcus faecalis and Staphylococcus aureus. On the basis of these findings, a fluorescence anisotropy-based high-throughput assay was developed and used successfully for identification of transglycosylase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A surface plasmon resonance analysis of the interaction between the antibiotic moenomycin A and penicillin-binding protein 1b.Chembiochem. 2002 Jun 3;3(6):559-65. doi: 10.1002/1439-7633(20020603)3:6<559::AID-CBIC559>3.0.CO;2-#. Chembiochem. 2002. PMID: 12325012
-
Targeting Bacterial Cell Wall Peptidoglycan Synthesis by Inhibition of Glycosyltransferase Activity.Chem Biol Drug Des. 2016 Feb;87(2):190-9. doi: 10.1111/cbdd.12662. Epub 2015 Oct 9. Chem Biol Drug Des. 2016. PMID: 26358369
-
Staphylococcus aureus Penicillin-Binding Protein 2 Can Use Depsi-Lipid II Derived from Vancomycin-Resistant Strains for Cell Wall Synthesis.Chemistry. 2013 Sep 2;19(36):12104-12. doi: 10.1002/chem.201301074. Epub 2013 Jul 19. Chemistry. 2013. PMID: 23873669 Free PMC article.
-
The molecular biology of moenomycins: towards novel antibiotics based on inhibition of bacterial peptidoglycan glycosyltransferases.Biol Chem. 2010 May;391(5):499-504. doi: 10.1515/BC.2010.053. Biol Chem. 2010. PMID: 20302515 Review.
-
Advances and prospects of analytic methods for bacterial transglycosylation and inhibitor discovery.Analyst. 2024 Apr 15;149(8):2204-2222. doi: 10.1039/d3an01968c. Analyst. 2024. PMID: 38517346 Review.
Cited by
-
Towards new antibiotics targeting bacterial transglycosylase: Synthesis of a Lipid II analog as stable transition-state mimic inhibitor.Bioorg Med Chem Lett. 2018 Sep 1;28(16):2708-2712. doi: 10.1016/j.bmcl.2018.03.035. Epub 2018 Mar 15. Bioorg Med Chem Lett. 2018. PMID: 29602680 Free PMC article.
-
Molecular basis and phenotype of methicillin resistance in Staphylococcus aureus and insights into new beta-lactams that meet the challenge.Antimicrob Agents Chemother. 2009 Oct;53(10):4051-63. doi: 10.1128/AAC.00084-09. Epub 2009 May 26. Antimicrob Agents Chemother. 2009. PMID: 19470504 Free PMC article. Review. No abstract available.
-
Tuning the moenomycin pharmacophore to enable discovery of bacterial cell wall synthesis inhibitors.J Am Chem Soc. 2013 Mar 13;135(10):3776-9. doi: 10.1021/ja4000933. Epub 2013 Mar 4. J Am Chem Soc. 2013. PMID: 23448584 Free PMC article.
-
Prospects for novel inhibitors of peptidoglycan transglycosylases.Bioorg Chem. 2014 Aug;55(100):16-26. doi: 10.1016/j.bioorg.2014.05.007. Epub 2014 May 21. Bioorg Chem. 2014. PMID: 24924926 Free PMC article. Review.
-
Efficient and versatile formation of glycosidic bonds via catalytic strain-release glycosylation with glycosyl ortho-2,2-dimethoxycarbonylcyclopropylbenzoate donors.Nat Commun. 2023 Jul 7;14(1):4010. doi: 10.1038/s41467-023-39619-7. Nat Commun. 2023. PMID: 37419914 Free PMC article.
References
-
- Silver LL. Novel inhibitors of bacterial cell wall synthesis. Curr Opin Microbiol. 2003;6:431–438. - PubMed
-
- Ostash B, Walker S. Bacterial TG inhibitors. Curr Opin Chem Biol. 2005;9:459–466. - PubMed
-
- Zehl M, Pittenauer E, Rizzi A, Allmaier G. Characterization of moenomycin antibiotic complex by multistage MALDI-IT/RTOF-MS and ESI-IT-MS. J Am Soc Mass Spectrom. 2006;17:1081–1090. - PubMed
-
- Eichhorn P, Aga DS. Characterization of moenomycin antibiotics from medicated chicken feed by ion-trap mass spectrometry with electrospray ionization. Rapid Commun Mass Spectrom. 2005;19:2179–2186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

