PD-1 and its ligands in tolerance and immunity
- PMID: 18173375
- PMCID: PMC10637733
- DOI: 10.1146/annurev.immunol.26.021607.090331
PD-1 and its ligands in tolerance and immunity
Abstract
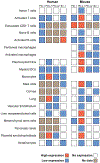
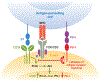
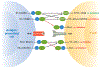
Programmed death 1 (PD-1) and its ligands, PD-L1 and PD-L2, deliver inhibitory signals that regulate the balance between T cell activation, tolerance, and immunopathology. Immune responses to foreign and self-antigens require specific and balanced responses to clear pathogens and tumors and yet maintain tolerance. Induction and maintenance of T cell tolerance requires PD-1, and its ligand PD-L1 on nonhematopoietic cells can limit effector T cell responses and protect tissues from immune-mediated tissue damage. The PD-1:PD-L pathway also has been usurped by microorganisms and tumors to attenuate antimicrobial or tumor immunity and facilitate chronic infection and tumor survival. The identification of B7-1 as an additional binding partner for PD-L1, together with the discovery of an inhibitory bidirectional interaction between PD-L1 and B7-1, reveals new ways the B7:CD28 family regulates T cell activation and tolerance. In this review, we discuss current understanding of the immunoregulatory functions of PD-1 and its ligands and their therapeutic potential.
Figures

Similar articles
-
The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo.J Immunol. 2011 Aug 1;187(3):1097-105. doi: 10.4049/jimmunol.1003496. Epub 2011 Jun 22. J Immunol. 2011. PMID: 21697456 Free PMC article.
-
Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells.Blood. 2007 Jul 1;110(1):186-92. doi: 10.1182/blood-2006-12-062422. Epub 2007 Mar 28. Blood. 2007. PMID: 17392506 Free PMC article.
-
Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production.J Immunol. 2003 Feb 1;170(3):1257-66. doi: 10.4049/jimmunol.170.3.1257. J Immunol. 2003. PMID: 12538684
-
The B7 family revisited.Annu Rev Immunol. 2005;23:515-48. doi: 10.1146/annurev.immunol.23.021704.115611. Annu Rev Immunol. 2005. PMID: 15771580 Review.
-
Role of PD-L1 and PD-L2 in allergic diseases and asthma.Allergy. 2011 Feb;66(2):155-62. doi: 10.1111/j.1398-9995.2010.02458.x. Epub 2010 Aug 17. Allergy. 2011. PMID: 20722638 Free PMC article. Review.
Cited by
-
A splicing isoform of PD-1 promotes tumor progression as a potential immune checkpoint.Nat Commun. 2024 Oct 23;15(1):9114. doi: 10.1038/s41467-024-53561-2. Nat Commun. 2024. PMID: 39438489 Free PMC article.
-
Association of gestational diabetes mellitus and negative modulation of the specific humoral and cellular immune response against Toxoplasma gondii.Front Immunol. 2022 Sep 20;13:925762. doi: 10.3389/fimmu.2022.925762. eCollection 2022. Front Immunol. 2022. PMID: 36203592 Free PMC article.
-
PD-1 deficiency enhances humoral immunity of malaria infection treatment vaccine.Infect Immun. 2015 May;83(5):2011-7. doi: 10.1128/IAI.02621-14. Epub 2015 Mar 2. Infect Immun. 2015. PMID: 25733520 Free PMC article.
-
Immune checkpoint inhibitors in malignant lymphoma: Advances and perspectives.Chin J Cancer Res. 2020 Jun;32(3):303-318. doi: 10.21147/j.issn.1000-9604.2020.03.03. Chin J Cancer Res. 2020. PMID: 32694896 Free PMC article.
-
Immune targeting of three independent suppressive pathways (TIGIT, PD-L1, TGFβ) provides significant antitumor efficacy in immune checkpoint resistant models.Oncoimmunology. 2022 Oct 1;11(1):2124666. doi: 10.1080/2162402X.2022.2124666. eCollection 2022. Oncoimmunology. 2022. PMID: 36211806 Free PMC article.
References
-
- Lafferty KJ, Cunningham AJ. 1975. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci 53:27–42 - PubMed
-
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541–47 - PubMed
-
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, et al. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985–88 - PubMed
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

