The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) therapy
- PMID: 18171609
- PMCID: PMC3337335
- DOI: 10.1016/j.bbi.2007.11.001
The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) therapy
Abstract
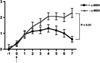
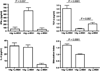
The neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) plays an important role in immune privilege by its suppression of inflammation, and its induction of regulatory T cells. This finding led us to test the possibility that we can use alpha-MSH to suppress autoimmune diseases, and promote re-establishment of immune tolerance to autoantigens. To test this possibility, SJL mice with experimental autoimmune encephalomyelitis (EAE) were injected with alpha-MSH at the first signs of paralysis. The alpha-MSH-treated mice in comparison with untreated EAE mice had a profound diminishment in the severity and tempo of EAE. The spleen cells in alpha-MSH-treated EAE produced TGF-beta in response to PLP-antigen stimulation in contrast to untreated mice spleen cells that produced IFN-gamma. When the alpha-MSH-treated EAE mice were reimmunized there was a delay of a week before the second episode of EAE. Although this delay maybe because of the induction of TGF-beta-producing spleen cells by the alpha-MSH-treatment, it was not adequate to suppress IFN-gamma-production by PLP-antigen stimulated spleen cells from untreated mice, nor able to suppress the eventual second episode of EAE. Therefore, the injection of alpha-MSH at the onset of paralysis is extremely effective in diminishing the severity and tempo of EAE, and the subsequent induction of potential PLP-specific Treg cells suggests that an alpha-MSH therapy could be attempted as part of a therapeutic regiment to impose immunoregulation and immunosuppression on an autoimmune disease of the central nervous system.
Figures


Similar articles
-
In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH).Immunol Cell Biol. 2001 Aug;79(4):358-67. doi: 10.1046/j.1440-1711.2001.01022.x. Immunol Cell Biol. 2001. PMID: 11488983
-
Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells.Gene Ther. 2007 Mar;14(5):383-95. doi: 10.1038/sj.gt.3302862. Epub 2006 Oct 26. Gene Ther. 2007. PMID: 17066098
-
SVα-MSH, a novel α-melanocyte stimulating hormone analog, ameliorates autoimmune encephalomyelitis through inhibiting autoreactive CD4(+) T cells activation.J Neuroimmunol. 2014 Apr 15;269(1-2):9-19. doi: 10.1016/j.jneuroim.2014.01.010. Epub 2014 Jan 30. J Neuroimmunol. 2014. PMID: 24518673
-
Tolerance in the absence of autoantigen.Endocr Metab Immune Disord Drug Targets. 2007 Sep;7(3):203-10. doi: 10.2174/187153007781662549. Endocr Metab Immune Disord Drug Targets. 2007. PMID: 17897047 Free PMC article. Review.
-
Multi-modal antigen specific therapy for autoimmunity.Int Rev Immunol. 2001 Oct;20(5):593-611. doi: 10.3109/08830180109045580. Int Rev Immunol. 2001. PMID: 11890614 Review.
Cited by
-
[Nle4, D-Phe7]-α-MSH Inhibits Toll-Like Receptor (TLR)2- and TLR4-Induced Microglial Activation and Promotes a M2-Like Phenotype.PLoS One. 2016 Jun 30;11(6):e0158564. doi: 10.1371/journal.pone.0158564. eCollection 2016. PLoS One. 2016. PMID: 27359332 Free PMC article.
-
α-MSH: a potential neuroprotective and immunomodulatory agent for the treatment of stroke.J Cereb Blood Flow Metab. 2011 Feb;31(2):606-13. doi: 10.1038/jcbfm.2010.130. Epub 2010 Aug 11. J Cereb Blood Flow Metab. 2011. PMID: 20700130 Free PMC article.
-
Applications of the role of α-MSH in ocular immune privilege.Adv Exp Med Biol. 2010;681:143-9. doi: 10.1007/978-1-4419-6354-3_12. Adv Exp Med Biol. 2010. PMID: 21222267 Free PMC article. Review.
-
Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research.Endocr Rev. 2012 Aug;33(4):623-51. doi: 10.1210/er.2011-1016. Epub 2012 Jun 26. Endocr Rev. 2012. PMID: 22736674 Free PMC article. Review.
-
Neuropeptides: keeping the balance between pathogen immunity and immune tolerance.Curr Opin Pharmacol. 2010 Aug;10(4):473-81. doi: 10.1016/j.coph.2010.03.003. Curr Opin Pharmacol. 2010. PMID: 20399708 Free PMC article. Review.
References
-
- Bohm M, Luger TA. Melanocortins in fibroblast biology—current update and future perspective for dermatology. Exp. Dermatol. 2004;13(Suppl. 4):16–21. - PubMed
-
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. Alpha-melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J. Biol. Chem. 2005;280:5795–5802. - PubMed
-
- Brzoska T, Kalden DH, Scholzen T, Luger TA. Molecular basis of the alpha-MSH/IL-1 antagonism. Ann. NY Acad. Sci. 1999;885:230–238. - PubMed
-
- Butler AA, Cone RD. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. - PubMed
-
- Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation. 1994;1:28–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

