Identification of somatic JAK1 mutations in patients with acute myeloid leukemia
- PMID: 18160671
- PMCID: PMC2343608
- DOI: 10.1182/blood-2007-05-090308
Identification of somatic JAK1 mutations in patients with acute myeloid leukemia
Abstract
Somatic mutations in JAK2 are frequently found in myeloproliferative diseases, and gain-of-function JAK3 alleles have been identified in M7 acute myeloid leukemia (AML), but a role for JAK1 in AML has not been described. We screened the entire coding region of JAK1 by total exonic resequencing of bone marrow DNA samples from 94 patients with de novo AML. We identified 2 novel somatic mutations in highly conserved residues of the JAK1 gene (T478S, V623A), in 2 separate patients and confirmed these by resequencing germ line DNA samples from the same patients. Overexpression of mutant JAK1 did not transform primary murine cells in standard assays, but compared with wild-type JAK1, JAK1(T478S), and JAK1(V623A) expression was associated with increased STAT1 activation in response to type I interferon and activation of multiple downstream signaling pathways. This is the first report to demonstrate somatic JAK1 mutations in AML and suggests that JAK1 mutations may function as disease-modifying mutations in AML pathogenesis.
Figures
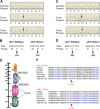
 ) consistently detected in both forward and reverse sequencing reactions but not present in the germline samples. (B,D) Change of amino acid sequences as a result of mutations. (E) Schematic diagram of JAK1 protein structure. Somatic mutations are indicated by arrows. The Thr478 residue resides in the β2 strand of the SH2 (JH3-JH4) domain near the phospho-tyrosine binding site of this domain. The Val623 residue resides in the β3 strand of the pseudo-kinase (JH2) domain in close proximity to the G-loop binding site of this domain. (F) Alignment of peptide sequences of conserved JAK1 residues. Both JAK1 mutations affect residues that are highly conserved throughout evolution.
) consistently detected in both forward and reverse sequencing reactions but not present in the germline samples. (B,D) Change of amino acid sequences as a result of mutations. (E) Schematic diagram of JAK1 protein structure. Somatic mutations are indicated by arrows. The Thr478 residue resides in the β2 strand of the SH2 (JH3-JH4) domain near the phospho-tyrosine binding site of this domain. The Val623 residue resides in the β3 strand of the pseudo-kinase (JH2) domain in close proximity to the G-loop binding site of this domain. (F) Alignment of peptide sequences of conserved JAK1 residues. Both JAK1 mutations affect residues that are highly conserved throughout evolution.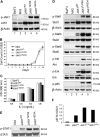
Comment in
-
Hitchhikers' guide to the leukemia genome.Blood. 2008 May 1;111(9):4428-9. doi: 10.1182/blood-2008-02-136952. Blood. 2008. PMID: 18441244 No abstract available.
Similar articles
-
Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia.Blood. 2008 May 1;111(9):4797-808. doi: 10.1182/blood-2007-09-113027. Epub 2008 Feb 12. Blood. 2008. PMID: 18270328 Free PMC article.
-
Absence of gain-of-function JAK1 and JAK3 mutations in adult T cell leukemia/lymphoma.Int J Hematol. 2010 Sep;92(2):320-5. doi: 10.1007/s12185-010-0653-2. Epub 2010 Aug 10. Int J Hematol. 2010. PMID: 20697856
-
Somatic mutations of JAK1 and JAK3 in acute leukemias and solid cancers.Clin Cancer Res. 2008 Jun 15;14(12):3716-21. doi: 10.1158/1078-0432.CCR-07-4839. Clin Cancer Res. 2008. PMID: 18559588
-
JAK and MPL mutations in myeloid malignancies.Leuk Lymphoma. 2008 Mar;49(3):388-97. doi: 10.1080/10428190801895360. Leuk Lymphoma. 2008. PMID: 18297515 Review.
-
[Pathogenesis and genetic landscape of acute myeloid leukemia].Magy Onkol. 2017 Mar 8;61(1):21-28. Epub 2016 Mar 20. Magy Onkol. 2017. PMID: 28273185 Review. Hungarian.
Cited by
-
The JAK-STAT pathway: impact on human disease and therapeutic intervention.Annu Rev Med. 2015;66:311-28. doi: 10.1146/annurev-med-051113-024537. Annu Rev Med. 2015. PMID: 25587654 Free PMC article. Review.
-
An Unbiased High-Throughput Screen to Identify Novel Effectors That Impact on Cardiomyocyte Aggregate Levels.Circ Res. 2017 Sep 1;121(6):604-616. doi: 10.1161/CIRCRESAHA.117.310945. Epub 2017 Jun 27. Circ Res. 2017. PMID: 28655832 Free PMC article.
-
JAK kinase targeting in hematologic malignancies: a sinuous pathway from identification of genetic alterations towards clinical indications.Haematologica. 2015 Oct;100(10):1240-53. doi: 10.3324/haematol.2015.132142. Haematologica. 2015. PMID: 26432382 Free PMC article. Review.
-
Janus kinases in immune cell signaling.Immunol Rev. 2009 Mar;228(1):273-87. doi: 10.1111/j.1600-065X.2008.00754.x. Immunol Rev. 2009. PMID: 19290934 Free PMC article. Review.
-
Transforming JAK1 mutations exhibit differential signalling, FERM domain requirements and growth responses to interferon-γ.Biochem J. 2010 Dec 1;432(2):255-65. doi: 10.1042/BJ20100774. Biochem J. 2010. PMID: 20868368 Free PMC article.
References
-
- Ihle JN. The Janus protein tyrosine kinases in hematopoietic cytokine signaling. Semin Immunol. 1995;7:247–254. - PubMed
-
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. - PubMed
-
- Benekli M, Baer MR, Baumann H, Wetzler M. Signal transducer and activator of transcription proteins in leukemias. Blood. 2003;101:2940–2954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

