NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis
- PMID: 18160433
- PMCID: PMC2259000
- DOI: 10.1128/JVI.02033-07
NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis
Abstract
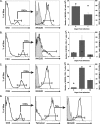
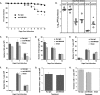
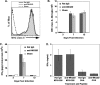
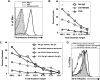
Inoculation with the neurotropic JHM strain of mouse hepatitis virus (JHMV) into the central nervous system (CNS) of mice results in an acute encephalitis associated with an immune-mediated demyelinating disease. During acute disease, infiltrating CD8(+) T cells secrete gamma interferon (IFN-gamma) that controls replication in oligodendrocytes, while infected astrocytes and microglia are susceptible to perforin-mediated lysis. The present study was undertaken to reveal the functional contributions of the activating NKG2D receptor in host defense and disease following JHMV infection. NKG2D ligands RAE-1, MULT1, and H60 were expressed within the CNS following JHMV infection. The immunophenotyping of infiltrating cells revealed that NKG2D was expressed on approximately 90% of infiltrating CD8(+) T cells during acute and chronic disease. Blocking NKG2D following JHMV infection resulted in increased mortality that correlated with increased viral titers within the CNS. Anti-NKG2D treatment did not alter T-cell infiltration into the CNS or the generation of virus-specific CD8(+) T cells, and the expression of IFN-gamma was not affected. However, cytotoxic T-lymphocyte (CTL) activity was dependent on NKG2D expression, because anti-NKG2D treatment resulted in a dramatic reduction in lytic activity by virus-specific CD8(+) T cells. Blocking NKG2D during chronic disease did not affect either T-cell or macrophage infiltration or the severity of demyelination, indicating that NKG2D does not contribute to virus-induced demyelination. These findings demonstrate a functional role for NKG2D in host defense during acute viral encephalitis by selectively enhancing CTL activity by infiltrating virus-specific CD8(+) T cells.
Figures


Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
4-1BB regulates NKG2D costimulation in human cord blood CD8+ T cells.Blood. 2008 Feb 1;111(3):1378-86. doi: 10.1182/blood-2007-01-069450. Epub 2007 Nov 16. Blood. 2008. PMID: 18024793 Free PMC article.
-
A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells.J Neuroinflammation. 2011 Aug 12;8:99. doi: 10.1186/1742-2094-8-99. J Neuroinflammation. 2011. PMID: 21838860 Free PMC article.
-
2B4 expression on natural killer cells increases in HIV-1 infected patients followed prospectively during highly active antiretroviral therapy.Clin Exp Immunol. 2005 Sep;141(3):526-33. doi: 10.1111/j.1365-2249.2005.02869.x. Clin Exp Immunol. 2005. PMID: 16045743 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
-
MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination.Stem Cells. 2012 Nov;30(11):2584-95. doi: 10.1002/stem.1234. Stem Cells. 2012. PMID: 22969049 Free PMC article.
-
The chemokine receptor CXCR2 and coronavirus-induced neurologic disease.Virology. 2013 Jan 5;435(1):110-7. doi: 10.1016/j.virol.2012.08.049. Virology. 2013. PMID: 23217621 Free PMC article. Review.
-
Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function.Clin Exp Immunol. 2014 Jan;175(1):79-91. doi: 10.1111/cei.12186. Clin Exp Immunol. 2014. PMID: 23937663 Free PMC article.
-
The pathogenesis of murine coronavirus infection of the central nervous system.Crit Rev Immunol. 2010;30(2):119-30. doi: 10.1615/critrevimmunol.v30.i2.20. Crit Rev Immunol. 2010. PMID: 20370625 Free PMC article. Review.
-
A protective role for ELR+ chemokines during acute viral encephalomyelitis.PLoS Pathog. 2009 Nov;5(11):e1000648. doi: 10.1371/journal.ppat.1000648. Epub 2009 Nov 6. PLoS Pathog. 2009. PMID: 19893623 Free PMC article.
References
-
- Agresti, A. 1992. A survey of exact interference for contingency tables. Stat. Sci. 7131-177.
-
- Aloisi, F., F. Ria, and L. Adorini. 2000. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol. Today 21141-147. - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1633379-3387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

