Dimeric PKD regulates membrane fission to form transport carriers at the TGN
- PMID: 18086912
- PMCID: PMC2140039
- DOI: 10.1083/jcb.200703166
Dimeric PKD regulates membrane fission to form transport carriers at the TGN
Abstract
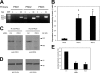
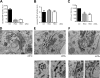
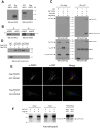
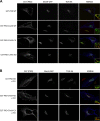
Protein kinase D (PKD) is recruited to the trans-Golgi network (TGN) through interaction with diacylglycerol (DAG) and is required for the biogenesis of TGN to cell surface transport carriers. We now provide definitive evidence that PKD has a function in membrane fission. PKD depletion by siRNA inhibits trafficking from the TGN, whereas expression of a constitutively active PKD converts TGN into small vesicles. These findings demonstrate that PKD regulates membrane fission and this activity is used to control the size of transport carriers, and to prevent uncontrolled vesiculation of TGN during protein transport.
Figures
Similar articles
-
PKD regulates membrane fission to generate TGN to cell surface transport carriers.Cold Spring Harb Perspect Biol. 2011 Feb 1;3(2):a005280. doi: 10.1101/cshperspect.a005280. Cold Spring Harb Perspect Biol. 2011. PMID: 21421913 Free PMC article. Review.
-
Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane.Science. 2002 Jan 11;295(5553):325-8. doi: 10.1126/science.1066759. Epub 2001 Nov 29. Science. 2002. PMID: 11729268
-
The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers.Cells. 2021 Jun 28;10(7):1618. doi: 10.3390/cells10071618. Cells. 2021. PMID: 34203456 Free PMC article. Review.
-
Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network.Nat Cell Biol. 2004 Feb;6(2):106-12. doi: 10.1038/ncb1090. Epub 2004 Jan 25. Nat Cell Biol. 2004. PMID: 14743217 Free PMC article.
-
Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain.EMBO J. 2001 Nov 1;20(21):5982-90. doi: 10.1093/emboj/20.21.5982. EMBO J. 2001. PMID: 11689438 Free PMC article.
Cited by
-
PKD controls mitotic Golgi complex fragmentation through a Raf-MEK1 pathway.Mol Biol Cell. 2013 Feb;24(3):222-33. doi: 10.1091/mbc.E12-03-0198. Epub 2012 Dec 14. Mol Biol Cell. 2013. PMID: 23242995 Free PMC article.
-
Recruitment of arfaptins to the trans-Golgi network by PI(4)P and their involvement in cargo export.EMBO J. 2013 Jun 12;32(12):1717-29. doi: 10.1038/emboj.2013.116. Epub 2013 May 21. EMBO J. 2013. PMID: 23695357 Free PMC article.
-
Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2.Mol Cell Biol. 2011 Feb;31(4):710-20. doi: 10.1128/MCB.01154-10. Epub 2010 Dec 20. Mol Cell Biol. 2011. PMID: 21173164 Free PMC article.
-
Defining the subcellular distribution and metabolic channeling of phosphatidylinositol.J Cell Biol. 2020 Mar 2;219(3):e201906130. doi: 10.1083/jcb.201906130. J Cell Biol. 2020. PMID: 32211894 Free PMC article.
-
RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells.Mol Syst Biol. 2012;8:629. doi: 10.1038/msb.2012.59. Mol Syst Biol. 2012. PMID: 23212246 Free PMC article.
References
-
- Bard, F., and V. Malhotra. 2006. The formation of TGN-to-plasma-membrane transport carriers. Annu. Rev. Cell Dev. Biol. 22:439–455. - PubMed
-
- Bard, F., L. Casano, A. Mallabiabarrena, E. Wallace, K. Saito, H. Kitayama, G. Guizzunti, Y. Hu, F. Wendler, R. Dasgupta, et al. 2006. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 439:604–607. - PubMed
-
- Baron, C.L., and V. Malhotra. 2002. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 295:325–328. - PubMed
-
- Choy, E., V.K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I.E. Ivanov, and M.R. Philips. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 98:69–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

