Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum
- PMID: 18083830
- PMCID: PMC2238148
- DOI: 10.1128/EC.00245-07
Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum
Abstract
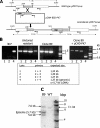
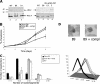
PfPK7 is an orphan protein kinase of Plasmodium falciparum with maximal homology to MEK3/6 and to fungal protein kinase A proteins in its C-terminal and N-terminal regions, respectively. We showed previously that recombinant PfPK7 is active on various substrates but is unable to phosphorylate the Plasmodium falciparum mitogen-activated protein kinase homologues, suggesting that it is not a MEK functional homologue. Using a reverse genetics approach to investigate the function of this enzyme in live parasites, we now show that PfPK7(-) parasite clones display phenotypes at two stages of their life cycle: first, a decrease in the rate of asexual growth in erythrocytes associated with a lower number of daughter merozoites generated per schizont, and second, a dramatic reduction in the ability to produce oocysts in the mosquito vector. A normal asexual growth rate and the ability to produce oocysts are restored if a functional copy of the PfPK7 gene is reintroduced into the PfPK7(-) parasites. Hence, PfPK7 is involved in a pathway that regulates parasite proliferation and development.
Figures
Similar articles
-
PfPK7, an atypical MEK-related protein kinase, reflects the absence of classical three-component MAPK pathways in the human malaria parasite Plasmodium falciparum.Mol Microbiol. 2005 Jan;55(1):184-96. doi: 10.1111/j.1365-2958.2004.04393.x. Mol Microbiol. 2005. PMID: 15612927
-
Characterization of Plasmodium falciparum Atypical Kinase PfPK7- Dependent Phosphoproteome.J Proteome Res. 2018 Jun 1;17(6):2112-2123. doi: 10.1021/acs.jproteome.8b00062. Epub 2018 Apr 30. J Proteome Res. 2018. PMID: 29678115
-
Ubiquitin proteasome system and the atypical kinase PfPK7 are involved in melatonin signaling in Plasmodium falciparum.J Pineal Res. 2012 Sep;53(2):147-53. doi: 10.1111/j.1600-079X.2012.00981.x. Epub 2012 Feb 21. J Pineal Res. 2012. PMID: 22348509 Free PMC article.
-
Role of Melatonin in the Synchronization of Asexual Forms in the Parasite Plasmodium falciparum.Biomolecules. 2020 Aug 27;10(9):1243. doi: 10.3390/biom10091243. Biomolecules. 2020. PMID: 32867164 Free PMC article. Review.
-
The cytoadherence linked asexual gene family of Plasmodium falciparum: are there roles other than cytoadherence?Int J Parasitol. 1999 Jun;29(6):939-44. doi: 10.1016/s0020-7519(99)00046-6. Int J Parasitol. 1999. PMID: 10480731 Review.
Cited by
-
How Many Is Enough? - Challenges of Multinucleated Cell Division in Malaria Parasites.Front Cell Infect Microbiol. 2021 May 7;11:658616. doi: 10.3389/fcimb.2021.658616. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34026661 Free PMC article. Review.
-
Nutrient sensing modulates malaria parasite virulence.Nature. 2017 Jul 13;547(7662):213-216. doi: 10.1038/nature23009. Epub 2017 Jul 5. Nature. 2017. PMID: 28678779 Free PMC article.
-
Plasmodium falciparum Development from Gametocyte to Oocyst: Insight from Functional Studies.Microorganisms. 2023 Jul 31;11(8):1966. doi: 10.3390/microorganisms11081966. Microorganisms. 2023. PMID: 37630530 Free PMC article. Review.
-
Melatonin activates FIS1, DYN1, and DYN2 Plasmodium falciparum related-genes for mitochondria fission: Mitoemerald-GFP as a tool to visualize mitochondria structure.J Pineal Res. 2019 Mar;66(2):e12484. doi: 10.1111/jpi.12484. Epub 2018 Oct 5. J Pineal Res. 2019. PMID: 29480948 Free PMC article.
-
In vitro and in silico assessment of new beta amino ketones with antiplasmodial activity.Rev Soc Bras Med Trop. 2022 Sep 26;55:e0590. doi: 10.1590/0037-8682-0590-2022. eCollection 2022. Rev Soc Bras Med Trop. 2022. PMID: 36169491 Free PMC article.
References
-
- Anamika, N. Srinivasan, and A. Krupa. 2005. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins 58180-189. - PubMed
-
- Bell, A. S., and L. C. Ranford-Cartwright. 2004. A real-time PCR assay for quantifying Plasmodium falciparum infections in the mosquito vector. Int. J. Parasitol. 34795-802. - PubMed
-
- Carter, R., L. Ranford-Cartwright, and P. Alano. 1993. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol. Biol. 2167-88. - PubMed
-
- Coulson, R. M., C. F. Curtis, P. D. Ready, N. Hill, and D. F. Smith. 1990. Amplification and analysis of human DNA present in mosquito bloodmeals. Med. Vet. Entomol. 4357-366. - PubMed
-
- de Koning-Ward, T., A. Oliveri, L. Bertuccini, A. Hood, F. Silvestrini, C. Charvalias, P. Berzosa-Diaz, G. Camarda, T. McElwain, T. Papenfuss, J. Healer, L. Baldassari, B. Crabb, P. Alano, and L. Ranford-Cartwright. 2008. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 67278-290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

