Agonist-selective mechanisms of GPCR desensitization
- PMID: 18059321
- PMCID: PMC2268061
- DOI: 10.1038/sj.bjp.0707604
Agonist-selective mechanisms of GPCR desensitization
Abstract
The widely accepted model of G protein-coupled receptor (GPCR) regulation describes a system where the agonist-activated receptors couple to G proteins to induce a cellular response, and are subsequently phosphorylated by a family of kinases called the G protein-coupled receptor kinases (GRKs). The GRK-phosphorylated receptor then acts as a substrate for the binding of a family of proteins called arrestins, which uncouple the receptor and G protein so desensitizing the agonist-induced response. Other kinases, principally the second messenger-dependent protein kinases, are also known to play a role in the desensitization of many GPCR responses. It is now clear that there are subtle and complex interactions between GRKs and second messenger-dependent protein kinases in the regulation of GPCR function. Functional selectivity describes the ability of agonists to stabilize different active conformations of the same GPCR. With regard to desensitization, distinct agonist-activated conformations of a GPCR could undergo different molecular mechanisms of desensitization. An example of this is the mu opioid receptor (MOPr), where the agonists morphine and [D-Ala(2),N-MePhe(4),Gly-ol(5)]enkephalin (DAMGO) induce desensitization of the MOPr by different mechanisms, largely protein kinase C (PKC)- or GRK-dependent, respectively. This can be best explained by supposing that these two agonists stabilize distinct conformations of the MOPr, which are nevertheless able to couple to the relevant G-proteins and produce similar responses, yet are sufficiently different to trigger different regulatory processes. There is evidence that other GPCRs also undergo agonist-selective desensitization, but the full therapeutic consequences of this phenomenon await further detailed study.
Figures

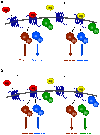

Similar articles
-
μ-Opioid receptor desensitization: homologous or heterologous?Eur J Neurosci. 2012 Dec;36(12):3636-42. doi: 10.1111/ejn.12003. Epub 2012 Sep 24. Eur J Neurosci. 2012. PMID: 23002724 Free PMC article.
-
Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells.Mol Pharmacol. 2006 Aug;70(2):676-85. doi: 10.1124/mol.106.022376. Epub 2006 May 8. Mol Pharmacol. 2006. PMID: 16682505
-
Role of G Protein-Coupled Receptor Kinases 2 and 3 in μ-Opioid Receptor Desensitization and Internalization.Mol Pharmacol. 2015 Aug;88(2):347-56. doi: 10.1124/mol.115.098293. Epub 2015 May 26. Mol Pharmacol. 2015. PMID: 26013542 Free PMC article.
-
Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling.Pharmacol Rev. 2001 Mar;53(1):1-24. Pharmacol Rev. 2001. PMID: 11171937 Review.
-
Desensitization of G protein-coupled receptors.Recent Prog Horm Res. 1996;51:319-51; discussion 352-3. Recent Prog Horm Res. 1996. PMID: 8701085 Review.
Cited by
-
Desensitization of human CRF2(a) receptor signaling governed by agonist potency and βarrestin2 recruitment.Regul Pept. 2013 Sep 10;186:62-76. doi: 10.1016/j.regpep.2013.06.009. Epub 2013 Jun 29. Regul Pept. 2013. PMID: 23820308 Free PMC article.
-
Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146.J Intern Med. 2017 Jan;281(1):25-40. doi: 10.1111/joim.12528. Epub 2016 Jun 16. J Intern Med. 2017. PMID: 27306986 Free PMC article. Review.
-
The spatial distribution of GPCR and Gβγ activity across a cell dictates PIP3 dynamics.Sci Rep. 2023 Feb 16;13(1):2771. doi: 10.1038/s41598-023-29639-0. Sci Rep. 2023. PMID: 36797332 Free PMC article.
-
Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm.Cell Mol Life Sci. 2011 Jan;68(1):1-13. doi: 10.1007/s00018-010-0465-9. Epub 2010 Aug 12. Cell Mol Life Sci. 2011. PMID: 20703892 Free PMC article. Review.
-
Regulation of heterologously expressed 5-HT1B receptors coupling to potassium channels in AtT-20 cells.Br J Pharmacol. 2019 Feb;176(3):451-465. doi: 10.1111/bph.14547. Epub 2018 Dec 28. Br J Pharmacol. 2019. PMID: 30447001 Free PMC article.
References
-
- Ally RA, Ives KL, Traube E, Eltounsi I, Chen PW, Cahill PJ, et al. Agonist- and protein kinase C-induced phosphorylation have similar functional consequences for gastrin-releasing peptide receptor signaling via Gq. Mol Pharmacol. 2003;64:890–904. - PubMed
-
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitisation of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol. 2004;66:1592–1598. - PubMed
-
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitisation and morphine tolerance. Trends Pharmacol Sci. 2006;27:558–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

