Cytoplasmic vacuolization responses to cytopathic bovine viral diarrhoea virus
- PMID: 18054406
- PMCID: PMC2289992
- DOI: 10.1016/j.virusres.2007.10.017
Cytoplasmic vacuolization responses to cytopathic bovine viral diarrhoea virus
Abstract
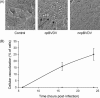
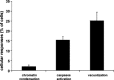
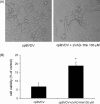
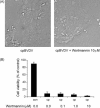
Bovine Viral Diarrhea Virus (BVDV) is a positive sense, single-stranded RNA virus which exhibits two biotypes in standard cell culture systems. The cytopathic strains of this virus (cpBVDV) induce dramatic cytoplasmic vacuolization in cell cultures, while infection with the non-cytopathic (NCP-BVDV) strains produces no overt changes in the host cells. Our results show that extensive cytoplasmic vacuolization is the earliest morphological change in response to cpBVDV infection in MDBK cells. Cells with extensive vacuolization showed no co-existing chromatin condensation, caspase activation, or loss of membrane integrity. In addition, the caspase inhibitor (zVAD-fmk), although improving cell viability of infected cells from 6.7+/-2.2% to 18.8+/-2.2%, did not prevent vacuolization. On the ultrastructural level, the virus-induced cytoplasmic vacuoles are single membrane structures containing organelles and cellular debris, which appear capable of fusing with other vacuoles and engulfing surrounding cytoplasmic materials. LysoTracker Red which marks lysosomes did not stain the virus-induced cytoplasmic vacuoles. In addition, this lysosomal dye could be observed in the cytoplasm of vacuolized cells, suggesting a lysosomal abnormality. Our data demonstrate that cpBVDV induced a novel cell death pathway in MDBK cells that is primarily associated with lysosomal dysfunction and the formation of phagocytic cytoplasmic vacuoles, and this mode of cell death is different from apoptosis and necrosis.
Figures




Similar articles
-
Protease 3C of hepatitis A virus induces vacuolization of lysosomal/endosomal organelles and caspase-independent cell death.BMC Cell Biol. 2015 Feb 27;16:4. doi: 10.1186/s12860-015-0050-z. BMC Cell Biol. 2015. PMID: 25886889 Free PMC article.
-
No caspase activation but overexpression of Bcl-2 in bovine cells infected with noncytopathic bovine virus diarrhoea virus.Vet Microbiol. 2003 Nov 7;96(4):313-26. doi: 10.1016/j.vetmic.2003.09.003. Vet Microbiol. 2003. PMID: 14599779
-
Autophagy induced by bovine viral diarrhea virus infection counteracts apoptosis and innate immune activation.Arch Virol. 2017 Oct;162(10):3103-3118. doi: 10.1007/s00705-017-3482-2. Epub 2017 Jul 12. Arch Virol. 2017. PMID: 28702931 Free PMC article.
-
[Bovine diarrhea virus: an update].Rev Argent Microbiol. 1997 Jan-Mar;29(1):47-61. Rev Argent Microbiol. 1997. PMID: 9229725 Review. Spanish.
-
Research advances on interferon (IFN) response during BVDV infection.Res Vet Sci. 2022 Dec;149:151-158. doi: 10.1016/j.rvsc.2022.04.011. Epub 2022 Jun 24. Res Vet Sci. 2022. PMID: 35839708 Review.
Cited by
-
Cytoplasmic vacuolization in cell death and survival.Oncotarget. 2016 Aug 23;7(34):55863-55889. doi: 10.18632/oncotarget.10150. Oncotarget. 2016. PMID: 27331412 Free PMC article. Review.
-
Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds.Iran J Vet Res. 2017 Summer;18(3):154-163. Iran J Vet Res. 2017. PMID: 29163643 Free PMC article. Review.
-
TNIK regulation of interferon signaling and endothelial cell response to virus infection.Front Cardiovasc Med. 2024 Jan 9;10:1213428. doi: 10.3389/fcvm.2023.1213428. eCollection 2023. Front Cardiovasc Med. 2024. PMID: 38264262 Free PMC article.
-
Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly.Viruses. 2016 Jun 7;8(6):160. doi: 10.3390/v8060160. Viruses. 2016. PMID: 27338443 Free PMC article. Review.
-
Protease 3C of hepatitis A virus induces vacuolization of lysosomal/endosomal organelles and caspase-independent cell death.BMC Cell Biol. 2015 Feb 27;16:4. doi: 10.1186/s12860-015-0050-z. BMC Cell Biol. 2015. PMID: 25886889 Free PMC article.
References
-
- Bampton E.T., Goemans C.G., Niranjan D., Mizushima N., Tolkovsky A.M. The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy. 2005;1:23–36. - PubMed
-
- Bendfeldt S., Grummer B., Greiser-Wilke I. No caspase activation but overexpression of Bcl-2 in bovine cells infected with noncytopathic bovine virus diarrhoea virus. Vet. Microbiol. 2003;96:313–326. - PubMed
-
- Bielefeldt Ohmann H., Bloch B. Electron microscopic studies of bovine viral diarrhea virus in tissues of diseased calves and in cell cultures. Arch. Virol. 1982;71:57–74. - PubMed
-
- Bolin S.R., McClurkin A.W., Cutlip R.C., Coria M.F. Severe clinical disease induced in cattle persistently infected with noncytopathic bovine viral diarrhea virus by superinfection with cytopathic bovine viral diarrhea virus. Am. J. Vet. Res. 1985;46:573–576. - PubMed
-
- Brownlie J. The pathways for bovine virus diarrhoea virus biotypes in the pathogenesis of disease. Arch. Virol. Suppl. 1991;3:79–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

