Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization
- PMID: 18045996
- PMCID: PMC2230606
- DOI: 10.1091/mbc.e06-01-0088
Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization
Abstract
In Rous sarcoma virus (RSV)-transformed baby hamster kidney (BHK) cells, invadopodia can self-organize into rings and belts, similarly to podosome distribution during osteoclast differentiation. The composition of individual invadopodia is spatiotemporally regulated and depends on invadopodia localization along the ring section: the actin core assembly precedes the recruitment of surrounding integrins and integrin-linked proteins, whereas the loss of the actin core was a prerequisite to invadopodia disassembly. We have shown that invadopodia ring expansion is controlled by paxillin phosphorylations on tyrosine 31 and 118, which allows invadopodia disassembly. In BHK-RSV cells, ectopic expression of the paxillin mutant Y31F-Y118F induces a delay in invadopodia disassembly and impairs their self-organization. A similar mechanism is unraveled in osteoclasts by using paxillin knockdown. Lack of paxillin phosphorylation, calpain or extracellular signal-regulated kinase inhibition, resulted in similar phenotype, suggesting that these proteins belong to the same regulatory pathways. Indeed, we have shown that paxillin phosphorylation promotes Erk activation that in turn activates calpain. Finally, we observed that invadopodia/podosomes ring expansion is required for efficient extracellular matrix degradation both in BHK-RSV cells and primary osteoclasts, and for transmigration through a cell monolayer.
Figures

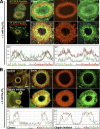
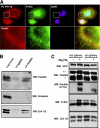
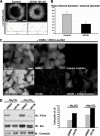
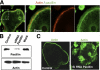
Similar articles
-
Vanadate-treated baby hamster kidney fibroblasts show cytoskeleton and adhesion patterns similar to their Rous sarcoma virus-transformed counterparts.J Cell Biochem. 1988 Jun;37(2):151-9. doi: 10.1002/jcb.240370203. J Cell Biochem. 1988. PMID: 2456294
-
Augmentation of invadopodia formation in temozolomide-resistant or adopted glioma is regulated by c-Jun terminal kinase-paxillin axis.Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):240-7. doi: 10.1016/j.bbrc.2015.10.122. Epub 2015 Oct 27. Biochem Biophys Res Commun. 2015. PMID: 26518652
-
Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia.J Cell Mol Med. 2010 Jun;14(6B):1569-93. doi: 10.1111/j.1582-4934.2009.00868.x. Epub 2009 Jul 28. J Cell Mol Med. 2010. PMID: 19656240 Free PMC article.
-
Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells.J Cell Biol. 1992 Dec;119(5):1309-25. doi: 10.1083/jcb.119.5.1309. J Cell Biol. 1992. PMID: 1447304 Free PMC article.
-
Signaling inputs to invadopodia and podosomes.J Cell Sci. 2013 Jul 15;126(Pt 14):2979-89. doi: 10.1242/jcs.079475. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843616 Free PMC article. Review.
Cited by
-
Physiological type I collagen organization induces the formation of a novel class of linear invadosomes.Mol Biol Cell. 2012 Jan;23(2):297-309. doi: 10.1091/mbc.E11-07-0594. Epub 2011 Nov 23. Mol Biol Cell. 2012. PMID: 22114353 Free PMC article.
-
Paxillin contracts the osteoclast cytoskeleton.J Bone Miner Res. 2012 Dec;27(12):2490-500. doi: 10.1002/jbmr.1706. J Bone Miner Res. 2012. PMID: 22807029 Free PMC article.
-
Spatiotemporal regulation of Src and its substrates at invadosomes.Eur J Cell Biol. 2012 Nov-Dec;91(11-12):878-88. doi: 10.1016/j.ejcb.2012.06.003. Epub 2012 Jul 22. Eur J Cell Biol. 2012. PMID: 22823952 Free PMC article. Review.
-
Coupling between acto-adhesive machinery and ECM degradation in invadosomes.Cell Adh Migr. 2014;8(3):256-62. doi: 10.4161/cam.28558. Cell Adh Migr. 2014. PMID: 24727371 Free PMC article. Review.
-
Regulation of sarcoma cell migration, invasion and invadopodia formation by AFAP1L1 through a phosphotyrosine-dependent pathway.Oncogene. 2016 Apr 21;35(16):2098-111. doi: 10.1038/onc.2015.272. Epub 2015 Jul 27. Oncogene. 2016. PMID: 26212012
References
-
- Abram C. L., Seals D. F., Pass I., Salinsky D., Maurer L., Roth T. M., Courtneidge S. A. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 2003;278:16844–16851. - PubMed
-
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. - PubMed
-
- Ayala I., Baldassarre M., Caldieri G., Buccione R. Invadopodia: a guided tour. Eur. J. Cell Biol. 2006;85:159–164. - PubMed
-
- Baldassarre M., Ayala I., Beznoussenko G., Giacchetti G., Machesky L. M., Luini A., Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur. J. Cell Biol. 2006;85:1217–1231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

