Orthotopic transplantation of immortalized mesencephalic progenitors (CSM14.1 cells) into the substantia nigra of hemiparkinsonian rats induces neuronal differentiation and motoric improvement
- PMID: 18036147
- PMCID: PMC2423384
- DOI: 10.1111/j.1469-7580.2007.00834.x
Orthotopic transplantation of immortalized mesencephalic progenitors (CSM14.1 cells) into the substantia nigra of hemiparkinsonian rats induces neuronal differentiation and motoric improvement
Abstract
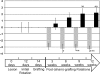
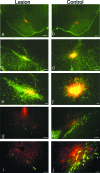
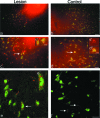
Neural progenitor cell grafting is a promising therapeutic option in the treatment of Parkinson's disease. In previous experiments we grafted temperature-sensitive immortalized CSM14.1 cells, derived from the ventral mesencephalon of E14-rats, bilaterally in the caudate putamen of adult hemiparkinsonian rats. In these studies we were not able to demonstrate either a therapeutic improvement or neuronal differentiation of transplanted cells. Here we examined whether CSM14.1 cells grafted bilaterally orthotopically in the substantia nigra of hemiparkinsonian rats have the potential to differentiate into dopaminergic neurons. Adult male rats received 6-hydroxydopamine into the right medial forebrain bundle, and successful lesions were evaluated with apomorphine-induced rotations 12 days after surgery. Two weeks after a successful lesion the animals received bilateral intranigral grafts consisting of either about 50 000 PKH26-labelled undifferentiated CSM14.1 cells (n = 16) or a sham-graft (n = 9). Rotations were evaluated 3, 6, 9 and 12 weeks post-grafting. Animals were finally perfused with 4% paraformaldehyde. Cryoprotected brain slices were prepared for immunohistochemistry using the freeze-thaw technique to preserve PKH26-labelling. Slices were immunostained against neuronal epitopes (NeuN, tyrosine hydroxylase) or glial fibrillary acidic protein. The CSM14.1-cell grafts significantly reduced the apomorphine-induced rotations 12 weeks post-grafting compared to the sham-grafts (P < 0.05). There was an extensive mediolateral migration (400-700 microm) of the PKH26-labelled cells within the host substantia nigra. Colocalization with NeuN or glial fibrillary acidic protein in transplanted cells was confirmed with confocal microscopy. No tyrosine hydroxylase-immunoreactive grafted cells were detectable. The therapeutic effect of the CSM14.1 cells could be explained either by their glial cell-derived neurotrophic factor-expression or their neural differentiation with positive effects on the basal ganglia neuronal networks.
Figures


Similar articles
-
Transplantation of immortalized mesencephalic progenitors (CSM14.1 cells) into the neonatal parkinsonian rat caudate putamen.J Neurosci Res. 2007 Mar;85(4):778-86. doi: 10.1002/jnr.21170. J Neurosci Res. 2007. PMID: 17203489
-
Enhancement of sensorimotor behavioral recovery in hemiparkinsonian rats with intrastriatal, intranigral, and intrasubthalamic nucleus dopaminergic transplants.J Neurosci. 2001 May 15;21(10):3521-30. doi: 10.1523/JNEUROSCI.21-10-03521.2001. J Neurosci. 2001. PMID: 11331381 Free PMC article.
-
Mesencephalic human neural progenitor cells transplanted into the neonatal hemiparkinsonian rat striatum differentiate into neurons and improve motor behaviour.J Anat. 2006 Dec;209(6):721-32. doi: 10.1111/j.1469-7580.2006.00654.x. J Anat. 2006. PMID: 17118060 Free PMC article.
-
Environmental cue-dependent dopaminergic neuronal differentiation and functional effect of grafted neuroepithelial stem cells in parkinsonian brain.Neurosurgery. 2009 Oct;65(4):741-53; discussion 753. doi: 10.1227/01.NEU.0000351281.45986.76. Neurosurgery. 2009. PMID: 19834380
-
Glial cells in intracerebral transplantation for Parkinson's disease.Neural Regen Res. 2020 Jul;15(7):1173-1178. doi: 10.4103/1673-5374.270296. Neural Regen Res. 2020. PMID: 31960796 Free PMC article. Review.
Cited by
-
Immortalization of neuronal progenitors using SV40 large T antigen and differentiation towards dopaminergic neurons.J Cell Mol Med. 2012 Nov;16(11):2592-610. doi: 10.1111/j.1582-4934.2012.01607.x. J Cell Mol Med. 2012. PMID: 22863662 Free PMC article. Review.
-
The proteome profiles of the olfactory bulb of juvenile, adult and aged rats - an ontogenetic study.Proteome Sci. 2015 Feb 15;13:8. doi: 10.1186/s12953-014-0058-x. eCollection 2015. Proteome Sci. 2015. PMID: 25709559 Free PMC article.
-
The proteome of the differentiating mesencephalic progenitor cell line CSM14.1 in vitro.Biomed Res Int. 2014;2014:351821. doi: 10.1155/2014/351821. Epub 2014 Jan 30. Biomed Res Int. 2014. PMID: 24592386 Free PMC article.
-
The Proteome Profiles of the Cerebellum of Juvenile, Adult and Aged Rats--An Ontogenetic Study.Int J Mol Sci. 2015 Sep 7;16(9):21454-85. doi: 10.3390/ijms160921454. Int J Mol Sci. 2015. PMID: 26370973 Free PMC article.
References
-
- Anton R, Kordower JH, Kane DJ, Markham CH, Bredesen DE. Neural transplantation of cells expressing the anti-apoptotic gene bcl-2. Cell Transpl. 1995;4:49–54. - PubMed
-
- Anton R, Kordower JH, Maidment NT, Manaster JS, Kane DJ, Rabizadeh S, et al. Neural-targeted gene therapy for rodent and primate hemiparkinsonism. Exp Neurol. 1994;127:207–218. - PubMed
-
- Björklund A. Dopaminergic transplants in experimental parkinsonism: cellular mechanisms of graft-induced functional recovery. Curr Opin Neurobiol. 1992;2:683–689. - PubMed
-
- Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. - PubMed
-
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, et al. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355:479–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

