Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response
- PMID: 18031569
- PMCID: PMC2253697
- DOI: 10.1111/j.1474-9726.2007.00357.x
Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response
Abstract
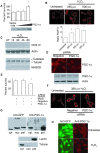
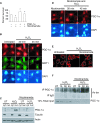
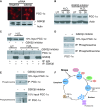
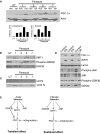
There is increasing evidence that longevity and stress resistance are connected, but the mechanism is unclear. We report that mitochondria are regulated in response to oxidative stress and calorie restriction through a shared mechanism involving peroxisome proliferator-activated receptor-gamma co-activator 1alpha (PGC-1alpha). We demonstrate that PGC-1alpha subcellular distribution is regulated, and its transcriptional activity is promoted through SIRT1-dependent nuclear accumulation. In addition, the duration of PGC-1alpha activity is regulated by glycogen synthase kinase beta (GSK3beta), which targets PGC-1alpha for intranuclear proteasomal degradation. This mechanism of regulation permits the rapidity and persistence of PGC-1alpha activation to be independently controlled. We provide evidence that this pathway of PGC-1alpha regulation occurs in vivo in mice, both in the oxidative stress response and with calorie restriction. Our data show how mitochondrial function may be adapted in response to external stimuli, and support the concept that such adaptation is critically involved in cellular survival and in lifespan extension by calorie restriction.
Figures

Similar articles
-
Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis.J Biol Chem. 2010 Jul 9;285(28):21590-9. doi: 10.1074/jbc.M109.070169. Epub 2010 May 6. J Biol Chem. 2010. PMID: 20448046 Free PMC article.
-
Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1.Nature. 2005 Mar 3;434(7029):113-8. doi: 10.1038/nature03354. Nature. 2005. PMID: 15744310
-
SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD.J Biol Chem. 2009 Aug 14;284(33):21872-21880. doi: 10.1074/jbc.M109.022749. Epub 2009 Jun 24. J Biol Chem. 2009. PMID: 19553684 Free PMC article.
-
Sirt1 and the Mitochondria.Mol Cells. 2016 Feb;39(2):87-95. doi: 10.14348/molcells.2016.2318. Epub 2016 Feb 2. Mol Cells. 2016. PMID: 26831453 Free PMC article. Review.
-
Effects of Dietary Restriction on PGC-1α Regulation in the Development of Age-associated Diseases.Curr Aging Sci. 2024;17(3):189-195. doi: 10.2174/0118746098301226240402051508. Curr Aging Sci. 2024. PMID: 38616758 Review.
Cited by
-
The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH-NQO1.Mol Cell Biol. 2013 Jul;33(13):2603-13. doi: 10.1128/MCB.01672-12. Epub 2013 May 6. Mol Cell Biol. 2013. PMID: 23648480 Free PMC article.
-
Adipocyte-Mineralocorticoid Receptor Alters Mitochondrial Quality Control Leading to Mitochondrial Dysfunction and Senescence of Visceral Adipose Tissue.Int J Mol Sci. 2021 Mar 12;22(6):2881. doi: 10.3390/ijms22062881. Int J Mol Sci. 2021. PMID: 33809055 Free PMC article.
-
Srebp-1c/Fgf21/Pgc-1α Axis Regulated by Leptin Signaling in Adipocytes-Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue.Nutrients. 2020 Jul 10;12(7):2054. doi: 10.3390/nu12072054. Nutrients. 2020. PMID: 32664386 Free PMC article.
-
Sildenafil for the Treatment of Alzheimer's Disease: A Systematic Review.J Alzheimers Dis Rep. 2020 Apr 22;4(1):91-106. doi: 10.3233/ADR-200166. J Alzheimers Dis Rep. 2020. PMID: 32467879 Free PMC article. Review.
-
Modelling age-related metabolic disorders in the mouse.Mamm Genome. 2014 Oct;25(9-10):487-96. doi: 10.1007/s00335-014-9539-6. Epub 2014 Aug 15. Mamm Genome. 2014. PMID: 25118634 Free PMC article. Review.
References
-
- Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005;17:855–868. - PubMed
-
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

