Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin
- PMID: 17991725
- PMCID: PMC2219303
- DOI: 10.1210/en.2007-0838
Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin
Abstract
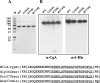
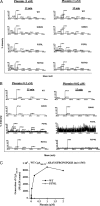
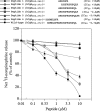
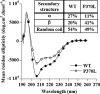
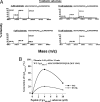
The plasma level of chromogranin A (CgA) is elevated in genetic hypertension. Conversely, the plasma level of the CgA peptide catestatin is diminished in individuals with established hypertension and those with a genetic risk of this disease. Resequencing of the human CHGA gene identified three naturally occurring variants of catestatin (Gly364Ser, Pro370Leu, and Arg374Gln) that exhibit different potencies in inhibiting catecholamine secretion. Here, we have examined whether there is any differential processing of the three CHGA variants to catestatin by the endoproteolytic enzyme plasmin. Plasmin digestion of the purified CgA proteins generated a stable biologically active 14-amino acid peptide (human CgA(360-373)) from the wild-type, Gly364Ser, and Arg374Gln proteins despite the disruption of the dibasic site (Arg(373)Arg(374)) in the Arg374Gln variant. Unexpectedly, the action of plasmin in generating the catestatin peptide from the Pro370Leu protein was less efficient. The efficiency of cleavage at the dibasic Arg(373) downward arrowArg(374) site in synthetic human CgA(360-380) was 3- to 4-fold less in Pro370Leu CgA, compared with the wild type. Circular dichroism of the synthetic CgA(352-372) suggested a difference in the amount of alpha-helix and beta-sheet between the wild-type and Pro370Leu CgA peptides. Because the Pro(370) residue is in the P4 position, the local secondary structure in the vicinity of the cleavage site may enforce the specificity or accessibility to plasmin. The less efficient proteolytic processing of the Pro370Leu protein by plasmin, coupled with the strong association of this variant with ethnicity, suggests that the Pro370Leu CHGA gene variant may contribute to the differential prevalence of cardiovascular disease across ethnic groups.
Figures

Similar articles
-
Cathepsin L colocalizes with chromogranin a in chromaffin vesicles to generate active peptides.Endocrinology. 2009 Aug;150(8):3547-57. doi: 10.1210/en.2008-1613. Epub 2009 Apr 16. Endocrinology. 2009. PMID: 19372204 Free PMC article.
-
Catecholamine release-inhibitory peptide catestatin (chromogranin A(352-372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension.Circulation. 2007 May 1;115(17):2271-81. doi: 10.1161/CIRCULATIONAHA.106.628859. Epub 2007 Apr 16. Circulation. 2007. PMID: 17438154
-
The catecholamine release-inhibitory "catestatin" fragment of chromogranin a: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses.Mol Pharmacol. 2004 Nov;66(5):1180-91. doi: 10.1124/mol.104.002139. Epub 2004 Aug 23. Mol Pharmacol. 2004. PMID: 15326220
-
Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure.Cardiovasc Res. 2008 Dec 1;80(3):330-8. doi: 10.1093/cvr/cvn155. Epub 2008 Jun 9. Cardiovasc Res. 2008. PMID: 18541522 Review.
-
Chromogranin A: a novel susceptibility gene for essential hypertension.Cell Mol Life Sci. 2010 Mar;67(6):861-74. doi: 10.1007/s00018-009-0208-y. Epub 2009 Nov 27. Cell Mol Life Sci. 2010. PMID: 19943077 Free PMC article. Review.
Cited by
-
Novel peptide isomer strategy for stable inhibition of catecholamine release: application to hypertension.Hypertension. 2012 Dec;60(6):1552-9. doi: 10.1161/HYPERTENSIONAHA.112.202127. Epub 2012 Nov 5. Hypertension. 2012. PMID: 23129699 Free PMC article.
-
A novel catestatin-induced antiadrenergic mechanism triggered by the endothelial PI3K-eNOS pathway in the myocardium.Cardiovasc Res. 2011 Sep 1;91(4):617-24. doi: 10.1093/cvr/cvr129. Epub 2011 May 4. Cardiovasc Res. 2011. PMID: 21543385 Free PMC article.
-
Glycosylated Chromogranin A: Potential Role in the Pathogenesis of Heart Failure.Curr Heart Fail Rep. 2017 Dec;14(6):478-488. doi: 10.1007/s11897-017-0360-x. Curr Heart Fail Rep. 2017. PMID: 28975588 Review.
-
Chromogranin/secretogranin proteins in murine heart: myocardial production of chromogranin A fragment catestatin (Chga(364-384)).Cell Tissue Res. 2010 Dec;342(3):353-61. doi: 10.1007/s00441-010-1059-4. Epub 2010 Nov 5. Cell Tissue Res. 2010. PMID: 21052719 Free PMC article.
-
Chromogranin A and its fragments as regulators of small intestinal neuroendocrine neoplasm proliferation.PLoS One. 2013 Nov 19;8(11):e81111. doi: 10.1371/journal.pone.0081111. eCollection 2013. PLoS One. 2013. PMID: 24260544 Free PMC article.
References
-
- Taupenot L, Harper KL, O’Connor DT 2003 Mechanisms of disease: the chromogranin-secretogranin family. N Engl J Med 348:1134–1149 - PubMed
-
- Takiyyuddin MA, Cervenka JH, Sullivan PA, Pandian MR, Parmer RJ, Barbosa JA, O’Connor DT 1990 Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation 81:185–195 - PubMed
-
- Reiffen FU, Gratzl M 1986 Ca2+ binding to chromaffin vesicle matrix proteins: effect of pH, Mg2+, and ionic strength. Biochemistry 25:4402–4406 - PubMed
-
- Videen JS, Mezger MS, Chang YM, O’Connor DT 1992 Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem 267:3066–3073 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL058120-099006/HL/NHLBI NIH HHS/United States
- P01 HL058120-09/HL/NHLBI NIH HHS/United States
- U01 HL069758/HL/NHLBI NIH HHS/United States
- U01 HL69758/HL/NHLBI NIH HHS/United States
- R01 DA011311/DA/NIDA NIH HHS/United States
- R29 DA011311/DA/NIDA NIH HHS/United States
- R01 DK060702-05/DK/NIDDK NIH HHS/United States
- P01 HL058120-090004/HL/NHLBI NIH HHS/United States
- R01 DK060702/DK/NIDDK NIH HHS/United States
- P01 HL058120/HL/NHLBI NIH HHS/United States
- DK60702/DK/NIDDK NIH HHS/United States
- P01 HL58120/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

