Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication
- PMID: 17989301
- PMCID: PMC6673253
- DOI: 10.1523/JNEUROSCI.2786-07.2007
Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication
Abstract
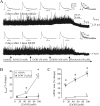
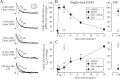
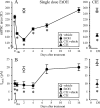
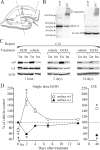
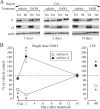
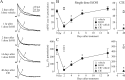
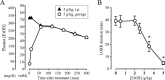
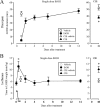
The time-dependent effects of ethanol (EtOH) intoxication on GABA(A) receptor (GABA(A)R) composition and function were studied in rats. A cross-linking assay and Western blot analysis of microdissected CA1 area of hippocampal slices obtained 1 h after EtOH intoxication (5 g/kg, gavage), revealed decreases in the cell-surface fraction of alpha4 and delta, but not alpha1, alpha5, or gamma2 GABA(A)R subunits, without changes in their total content. This was accompanied (in CA1 neuron recordings) by decreased magnitude of the picrotoxin-sensitive tonic current (I(tonic)), but not miniature IPSCs (mIPSCs), and by reduced enhancement of I(tonic) by EtOH, but not by diazepam. By 48 h after EtOH dosing, cell-surface alpha4 (80%) and gamma2 (82%) subunit content increased, and cell-surface alpha1 (-50%) and delta (-79%) and overall content were decreased. This was paralleled by faster decay of mIPSCs, decreased diazepam enhancement of both mIPSCs and I(tonic), and paradoxically increased mIPSC responsiveness to EtOH (10-100 mm). Sensitivity to isoflurane- or diazepam-induced loss of righting reflex was decreased at 12 and 24 h after EtOH intoxication, respectively, suggesting functional GABA(A)R tolerance. The plastic GABA(A)R changes were gradually and fully reversible by 2 weeks after single EtOH dosing, but unexplainably persisted long after withdrawal from chronic intermittent ethanol treatment, which leads to signs of alcohol dependence. Our data suggest that early tolerance to EtOH may result from excessive activation and subsequent internalization of alpha4betadelta extrasynaptic GABA(A)Rs. This leads to transcriptionally regulated increases in alpha4 and gamma2 and decreases in alpha1 subunits, with preferential insertion of the newly formed alpha4betagamma2 GABA(A)Rs at synapses.
Figures

Similar articles
-
Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons.Mol Pharmacol. 2011 Mar;79(3):432-42. doi: 10.1124/mol.110.068650. Epub 2010 Dec 16. Mol Pharmacol. 2011. PMID: 21163967 Free PMC article.
-
Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala.Neurochem Res. 2014 Jun;39(6):1162-70. doi: 10.1007/s11064-014-1297-z. Epub 2014 Apr 8. Neurochem Res. 2014. PMID: 24710789 Free PMC article.
-
Subunit Compensation and Plasticity of Synaptic GABA(A) Receptors Induced by Ethanol in α4 Subunit Knockout Mice.Front Neurosci. 2011 Sep 23;5:110. doi: 10.3389/fnins.2011.00110. eCollection 2011. Front Neurosci. 2011. PMID: 21977012 Free PMC article.
-
GABAA Receptor Plasticity in Alcohol Withdrawal.In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. PMID: 22787640 Free Books & Documents. Review.
-
Role of GABAA receptors in alcohol use disorders suggested by chronic intermittent ethanol (CIE) rodent model.Mol Brain. 2017 Sep 20;10(1):45. doi: 10.1186/s13041-017-0325-8. Mol Brain. 2017. PMID: 28931433 Free PMC article. Review.
Cited by
-
Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors.J Neurosci. 2012 Dec 5;32(49):17874-81. doi: 10.1523/JNEUROSCI.2535-12.2012. J Neurosci. 2012. PMID: 23223306 Free PMC article.
-
Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cgamma-dependent mechanism in cultured cerebral cortical neurons.Mol Pharmacol. 2010 May;77(5):793-803. doi: 10.1124/mol.109.063016. Epub 2010 Feb 16. Mol Pharmacol. 2010. PMID: 20159950 Free PMC article.
-
Sex differences in GABAA receptor subunit transcript expression are mediated by genotype in subjects with alcohol-related cirrhosis of the liver.Genes Brain Behav. 2022 Apr;21(4):e12785. doi: 10.1111/gbb.12785. Epub 2022 Mar 18. Genes Brain Behav. 2022. PMID: 35301805 Free PMC article.
-
The Yin and Yang of GABAergic and Glutamatergic Synaptic Plasticity: Opposites in Balance by Crosstalking Mechanisms.Front Synaptic Neurosci. 2022 May 19;14:911020. doi: 10.3389/fnsyn.2022.911020. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35663370 Free PMC article. Review.
-
Identification of gene expression profile in the rat brain resulting from acute alcohol intoxication.Mol Biol Rep. 2014 Dec;41(12):8303-17. doi: 10.1007/s11033-014-3731-3. Epub 2014 Sep 14. Mol Biol Rep. 2014. Retraction in: Mol Biol Rep. 2015 Jul;42(7):1241. doi: 10.1007/s11033-015-3873-y PMID: 25218841 Retracted.
References
-
- Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABAA receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem. 2001;79:1100–1108. - PubMed
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. - PubMed
-
- Bayard M, McIntyre J, Hill KR, Woodside J., Jr Alcohol withdrawal syndrome. Am Fam Physician. 2004;69:1443–1450. - PubMed
-
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. - PubMed
-
- Bieda MC, MacIver MB. Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol. 2004;92:1658–1667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
