Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions
- PMID: 17979681
- PMCID: PMC2268649
- DOI: 10.2174/156652307782151498
Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions
Abstract
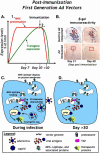
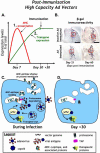
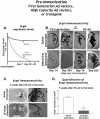
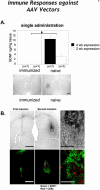
Researchers have conducted numerous pre-clinical and clinical gene transfer studies using recombinant viral vectors derived from a wide range of pathogenic viruses such as adenovirus, adeno-associated virus, and lentivirus. As viral vectors are derived from pathogenic viruses, they have an inherent ability to induce a vector specific immune response when used in vivo. The role of the immune response against the viral vector has been implicated in the inconsistent and unpredictable translation of pre-clinical success into therapeutic efficacy in human clinical trials using gene therapy to treat neurological disorders. Herein we thoroughly examine the effects of the innate and adaptive immune responses on therapeutic gene expression mediated by adenoviral, AAV, and lentiviral vectors systems in both pre-clinical and clinical experiments. Furthermore, the immune responses against gene therapy vectors and the resulting loss of therapeutic gene expression are examined in the context of the architecture and neuroanatomy of the brain immune system. The chapter closes with a discussion of the relationship between the elimination of transgene expression and the in vivo immunological synapses between immune cells and target virally infected brain cells. Importantly, although systemic immune responses against viral vectors injected systemically has thought to be deleterious in a number of trials, results from brain gene therapy clinical trials do not support this general conclusion suggesting brain gene therapy may be safer from an immunological standpoint.
Figures
Similar articles
-
Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy.Gene Ther. 2003 Jun;10(11):955-63. doi: 10.1038/sj.gt.3302037. Gene Ther. 2003. PMID: 12756416 Review.
-
Progress and prospects: immune responses to viral vectors.Gene Ther. 2010 Mar;17(3):295-304. doi: 10.1038/gt.2009.148. Epub 2009 Nov 12. Gene Ther. 2010. PMID: 19907498 Free PMC article. Review.
-
Immune responses to lentiviral vectors.Curr Gene Ther. 2007 Oct;7(5):306-15. doi: 10.2174/156652307782151515. Curr Gene Ther. 2007. PMID: 17979677 Review.
-
Immunology of neurological gene therapy: how T cells modulate viral vector-mediated therapeutic transgene expression through immunological synapses.Neurotherapeutics. 2007 Oct;4(4):715-24. doi: 10.1016/j.nurt.2007.07.010. Neurotherapeutics. 2007. PMID: 17920552 Free PMC article. Review.
-
AAV as an immunogen.Curr Gene Ther. 2007 Oct;7(5):325-33. doi: 10.2174/156652307782151416. Curr Gene Ther. 2007. PMID: 17979679 Review.
Cited by
-
Exogenous fms-like tyrosine kinase 3 ligand overrides brain immune privilege and facilitates recognition of a neo-antigen without causing autoimmune neuropathology.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14443-8. doi: 10.1073/pnas.0913496107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660723 Free PMC article.
-
MicroRNA-Regulated Gene Delivery Systems for Research and Therapeutic Purposes.Molecules. 2018 Jun 21;23(7):1500. doi: 10.3390/molecules23071500. Molecules. 2018. PMID: 29933586 Free PMC article. Review.
-
There must be a way out of here: identifying a safe and efficient combination of promoter, transgene, and vector backbone for gene therapy of neurological disease.Mol Ther. 2014 Feb;22(2):246-247. doi: 10.1038/mt.2013.297. Mol Ther. 2014. PMID: 24487564 Free PMC article. No abstract available.
-
Gene delivery to the nervous system.Mol Ther. 2008 Apr;16(4):640-6. doi: 10.1038/mt.2008.42. Mol Ther. 2008. PMID: 18362921 Free PMC article. No abstract available.
-
Getting miRNA Therapeutics into the Target Cells for Neurodegenerative Diseases: A Mini-Review.Front Mol Neurosci. 2016 Nov 22;9:129. doi: 10.3389/fnmol.2016.00129. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27920668 Free PMC article. Review.
References
-
- Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, Puntel M, Cheng Q, Prieto J, Ribas A, Kupiec-Weglinski J, van Rooijen N, Lassmann H, Lowenstein PR, Castro MG. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. - PMC - PubMed
-
- Barcia C, Gerdes C, Xiong W, Thomas CE, Liu C, Kroeger KM, Castro MG, Lowenstein PR. Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells may be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glial. Biology. 2006a;2(4):309–327. - PMC - PubMed
-
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, Kupiec-Weglinski J, Klatzmann D, Castro MG, Lowenstein PR. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J. Exp. Med. 2006b;203(9):2095–2107. - PMC - PubMed
-
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. - PubMed
-
- Blacklow NR, Hoggan MD, Rowe WP. Serologic evidence for human infection with adenovirus-associated viruses. J. Natl. Cancer Inst. 1968;40(2):319–327. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS042893-01A1/NS/NINDS NIH HHS/United States
- R21 NS054143-01A2/NS/NINDS NIH HHS/United States
- R21 NS047298/NS/NINDS NIH HHS/United States
- R01 NS044556/NS/NINDS NIH HHS/United States
- U01 NS052465/NS/NINDS NIH HHS/United States
- 1 R01 NS44556-01/NS/NINDS NIH HHS/United States
- NS445561-01/NS/NINDS NIH HHS/United States
- R01 NS044556-01/NS/NINDS NIH HHS/United States
- 1 U01 NS052465-01/NS/NINDS NIH HHS/United States
- 1 R21 NS047298-01/NS/NINDS NIH HHS/United States
- R01 NS042893/NS/NINDS NIH HHS/United States
- R03 TW006273-01A1/TW/FIC NIH HHS/United States
- R03 TW006273/TW/FIC NIH HHS/United States
- R21 NS047298-01/NS/NINDS NIH HHS/United States
- R21 NS054143/NS/NINDS NIH HHS/United States
- U54 NS045309-010005/NS/NINDS NIH HHS/United States
- U54 NS045309-01/NS/NINDS NIH HHS/United States
- R01 NS054193/NS/NINDS NIH HHS/United States
- U01 NS052465-01A2/NS/NINDS NIH HHS/United States
- 1 R21 NS054143-01/NS/NINDS NIH HHS/United States
- U54 NS045309/NS/NINDS NIH HHS/United States
- 1 R01 NS054193-01/NS/NINDS NIH HHS/United States
- 1 R03 TW006273-01/TW/FIC NIH HHS/United States
- R01 NS42893-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

