Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1
- PMID: 17977974
- PMCID: PMC2224571
- DOI: 10.1128/JVI.01305-07
Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1
Abstract
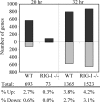
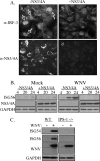
RIG-I and MDA5, two related pathogen recognition receptors (PRRs), are known to be required for sensing various RNA viruses. Here we investigated the roles that RIG-I and MDA5 play in eliciting the antiviral response to West Nile virus (WNV). Functional genomics analysis of WNV-infected fibroblasts from wild-type mice and RIG-I null mice revealed that the normal antiviral response to this virus occurs in two distinct waves. The initial response to WNV resulted in the expression of interferon (IFN) regulatory factor 3 target genes and IFN-stimulated genes, including several subtypes of alpha IFN. Subsequently, a second phase of IFN-dependent antiviral gene expression occurred very late in infection. In cells lacking RIG-I, both the initial and the secondary responses to WNV were delayed, indicating that RIG-I plays a critical role in initiating innate immunity against WNV. However, another PRR(s) was able to trigger a response to WNV in the absence of RIG-I. Disruption of both MDA5 and RIG-I pathways abrogated activation of the antiviral response to WNV, suggesting that MDA5 is involved in the host's defense against WNV infection. In addition, ablation of the function of IPS-1, an essential RIG-I and MDA5 adaptor molecule, completely disabled the innate antiviral response to WNV. Our data indicate that RIG-I and MDA5 are responsible for triggering downstream gene expression in response to WNV infection by signaling through IPS-1. We propose a model in which RIG-I and MDA5 operate cooperatively to establish an antiviral state and mediate an IFN amplification loop that supports immune effector gene expression during WNV infection.
Figures

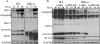


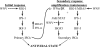
Similar articles
-
The essential, nonredundant roles of RIG-I and MDA5 in detecting and controlling West Nile virus infection.J Virol. 2013 Nov;87(21):11416-25. doi: 10.1128/JVI.01488-13. Epub 2013 Aug 21. J Virol. 2013. PMID: 23966395 Free PMC article.
-
Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity.J Virol. 2008 Jan;82(1):335-45. doi: 10.1128/JVI.01080-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942531 Free PMC article.
-
West Nile Virus NS1 Antagonizes Interferon Beta Production by Targeting RIG-I and MDA5.J Virol. 2017 Aug 24;91(18):e02396-16. doi: 10.1128/JVI.02396-16. Print 2017 Sep 15. J Virol. 2017. PMID: 28659477 Free PMC article.
-
The innate immune playbook for restricting West Nile virus infection.Viruses. 2013 Oct 30;5(11):2643-58. doi: 10.3390/v5112643. Viruses. 2013. PMID: 24178712 Free PMC article. Review.
-
[Role of IPS-1 in type I IFN induction].Nihon Rinsho. 2006 Jul;64(7):1231-5. Nihon Rinsho. 2006. PMID: 16841392 Review. Japanese.
Cited by
-
Immune responses of ducks infected with duck Tembusu virus.Front Microbiol. 2015 May 8;6:425. doi: 10.3389/fmicb.2015.00425. eCollection 2015. Front Microbiol. 2015. PMID: 26005441 Free PMC article.
-
Mosquito-borne flaviviruses and type I interferon: catch me if you can!Front Microbiol. 2023 Oct 30;14:1257024. doi: 10.3389/fmicb.2023.1257024. eCollection 2023. Front Microbiol. 2023. PMID: 37965539 Free PMC article. Review.
-
Central roles of NLRs and inflammasomes in viral infection.Nat Rev Immunol. 2010 Oct;10(10):688-98. doi: 10.1038/nri2851. Epub 2010 Sep 17. Nat Rev Immunol. 2010. PMID: 20847744 Free PMC article. Review.
-
Measure and countermeasure: type I IFN (IFN-alpha/beta) antiviral response against West Nile virus.J Innate Immun. 2009;1(5):435-45. doi: 10.1159/000226248. Epub 2009 Jun 24. J Innate Immun. 2009. PMID: 20375601 Free PMC article. Review.
-
A Balancing Act: MDA5 in Antiviral Immunity and Autoinflammation.Trends Microbiol. 2019 Jan;27(1):75-85. doi: 10.1016/j.tim.2018.08.007. Epub 2018 Sep 7. Trends Microbiol. 2019. PMID: 30201512 Free PMC article. Review.
References
-
- Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 2259-71. - PubMed
-
- Barnes, B. J., J. Richards, M. Mancl, S. Hanash, L. Beretta, and P. M. Pitha. 2004. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 27945194-45207. - PubMed
-
- Brazma, A., P. Hingamp, J. Quackenbush, G. Sherlock, P. Spellman, C. Stoeckert, J. Aach, W. Ansorge, C. A. Ball, H. C. Causton, T. Gaasterland, P. Glenisson, F. C. Holstege, I. F. Kim, V. Markowitz, J. C. Matese, H. Parkinson, A. Robinson, U. Sarkans, S. Schulze-Kremer, J. Stewart, R. Taylor, J. Vilo, and M. Vingron. 2001. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat. Genet. 29365-371. - PubMed
-
- Childs, K., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

