Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis
- PMID: 17971906
- PMCID: PMC2040213
- DOI: 10.1593/neo.07559
Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis
Abstract
In a mouse model of established extrahepatic colorectal metastasis, we analyzed whether stromal cell-derived factor (SDF) 1 stimulates tumor cell migration in vitro and angiogenesis and tumor growth in vivo.
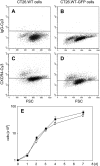
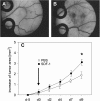
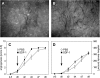
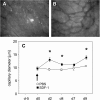
Methods: Using chemotaxis chambers, CT26.WT colorectal tumor cell migration was studied under stimulation with different concentrations of SDF-1. To evaluate angiogenesis and tumor growth in vivo, green fluorescent protein-transfected CT26.WT cells were implanted in dorsal skinfold chambers of syngeneic BALB/c mice. After 5 days, tumors were locally exposed to SDF-1. Cell proliferation, tumor microvascularization, and growth were studied during a further 9-day period using intravital fluorescence microscopy, histology, and immunohistochemistry. Tumors exposed to PBS only served as controls.
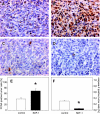
Results: In vitro, > 30% of unstimulated CT26.WT cells showed expression of the SDF-1 receptor CXCR4. On chemotaxis assay, SDF-1 provoked a dose-dependent increase in cell migration. In vivo, SDF-1 accelerated neovascularization and induced a significant increase in tumor growth. Capillaries of SDF-1-treated tumors showed significant dilation. Of interest, SDF-1 treatment was associated with a significantly increased expression of proliferating cell nuclear antigen and a downregulation of cleaved caspase-3.
Conclusion: Our study indicates that the CXC chemokine SDF-1 promotes tumor cell migration in vitro and tumor growth of established extrahepatic metastasis in vivo due to angiogenesis-dependent induction of tumor cell proliferation and inhibition of apoptotic cell death.
Keywords: Cancer; SDF-1; angiogenesis; chemokine; metastasis.
Figures
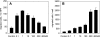
Similar articles
-
CXCR4 and CXCR7 regulate angiogenesis and CT26.WT tumor growth independent from SDF-1.Int J Cancer. 2010 Mar 15;126(6):1302-15. doi: 10.1002/ijc.24956. Int J Cancer. 2010. PMID: 19821487
-
Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer.Clin Exp Metastasis. 2014 Apr;31(4):447-59. doi: 10.1007/s10585-014-9639-4. Epub 2014 Feb 4. Clin Exp Metastasis. 2014. PMID: 24493023
-
Macrophage inflammatory protein-2 promotes angiogenesis, cell migration, and tumor growth in hepatic metastasis.Ann Surg Oncol. 2006 Feb;13(2):263-75. doi: 10.1245/ASO.2006.03.096. Epub 2006 Jan 20. Ann Surg Oncol. 2006. PMID: 16424980
-
The significance of the SDF-1/CXCR4 signaling pathway in the normal development.Mol Biol Rep. 2022 Apr;49(4):3307-3320. doi: 10.1007/s11033-021-07069-3. Epub 2022 Jan 24. Mol Biol Rep. 2022. PMID: 35067815 Review.
-
[Advance of research on SDF-1/CXCR4 axis and angiogenesis in leukemia--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008 Apr;16(2):447-51. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008. PMID: 18426684 Review. Chinese.
Cited by
-
Geraniol Suppresses Angiogenesis by Downregulating Vascular Endothelial Growth Factor (VEGF)/VEGFR-2 Signaling.PLoS One. 2015 Jul 8;10(7):e0131946. doi: 10.1371/journal.pone.0131946. eCollection 2015. PLoS One. 2015. PMID: 26154255 Free PMC article.
-
Phenotypic Knockout of CXCR4 Expression by a Novel Intrakine Mutant hSDF-1α/54/KDEL Inhibits Breast Cancer Metastasis.J Interferon Cytokine Res. 2015 Oct;35(10):771-8. doi: 10.1089/jir.2014.0141. Epub 2015 May 15. J Interferon Cytokine Res. 2015. PMID: 25978539 Free PMC article.
-
Changes in CXCL12/CXCR4-chemokine expression during onset of colorectal malignancies.Tumour Biol. 2011 Feb;32(1):189-96. doi: 10.1007/s13277-010-0112-y. Epub 2010 Sep 24. Tumour Biol. 2011. PMID: 20865359
-
Arylsulfonamide 64B Inhibits Hypoxia/HIF-Induced Expression of c-Met and CXCR4 and Reduces Primary Tumor Growth and Metastasis of Uveal Melanoma.Clin Cancer Res. 2019 Apr 1;25(7):2206-2218. doi: 10.1158/1078-0432.CCR-18-1368. Epub 2018 Dec 18. Clin Cancer Res. 2019. PMID: 30563937 Free PMC article.
-
Imaging ligand-dependent activation of CXCR7.Neoplasia. 2009 Oct;11(10):1022-35. doi: 10.1593/neo.09724. Neoplasia. 2009. PMID: 19794961 Free PMC article.
References
-
- Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Sakamoto M, Fukuda H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999;86:332–337. - PubMed
-
- Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231–255. - PubMed
-
- Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, Malka D, Pignon JP, Lasser P. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12:900–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
