STAGA recruits Mediator to the MYC oncoprotein to stimulate transcription and cell proliferation
- PMID: 17967894
- PMCID: PMC2223283
- DOI: 10.1128/MCB.01402-07
STAGA recruits Mediator to the MYC oncoprotein to stimulate transcription and cell proliferation
Abstract
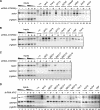
Activation of eukaryotic gene transcription involves the recruitment by DNA-binding activators of multiprotein histone acetyltransferase (HAT) and Mediator complexes. How these coactivator complexes functionally cooperate and the roles of the different subunits/modules remain unclear. Here we report physical interactions between the human HAT complex STAGA (SPT3-TAF9-GCN5-acetylase) and a "core" form of the Mediator complex during transcription activation by the MYC oncoprotein. Knockdown of the STAF65gamma component of STAGA in human cells prevents the stable association of TRRAP and GCN5 with the SPT3 and TAF9 subunits; impairs transcription of MYC-dependent genes, including MYC transactivation of the telomerase reverse transcriptase (TERT) promoter; and inhibits proliferation of MYC-dependent cells. STAF65gamma is required for SPT3/STAGA interaction with core Mediator and for MYC recruitment of SPT3, TAF9, and core Mediator components to the TERT promoter but is dispensable for MYC recruitment of TRRAP, GCN5, and p300 and for acetylation of nucleosomes and loading of TFIID and RNA polymerase II on the promoter. These results suggest a novel STAF65gamma-dependent function of STAGA-type complexes in cell proliferation and transcription activation by MYC postloading of TFIID and RNA polymerase II that involves direct recruitment of core Mediator.
Figures

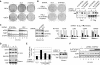


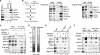
Similar articles
-
MYC interacts with the human STAGA coactivator complex via multivalent contacts with the GCN5 and TRRAP subunits.Biochim Biophys Acta. 2014 May;1839(5):395-405. doi: 10.1016/j.bbagrm.2014.03.017. Epub 2014 Apr 3. Biochim Biophys Acta. 2014. PMID: 24705139 Free PMC article.
-
c-Myc transformation domain recruits the human STAGA complex and requires TRRAP and GCN5 acetylase activity for transcription activation.J Biol Chem. 2003 May 30;278(22):20405-12. doi: 10.1074/jbc.M211795200. Epub 2003 Mar 26. J Biol Chem. 2003. PMID: 12660246 Free PMC article.
-
Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes.Hum Mol Genet. 2004 Jun 15;13(12):1257-65. doi: 10.1093/hmg/ddh139. Epub 2004 Apr 28. Hum Mol Genet. 2004. PMID: 15115762
-
Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation.Oncogene. 2007 Aug 13;26(37):5341-57. doi: 10.1038/sj.onc.1210604. Oncogene. 2007. PMID: 17694077 Review.
-
SAGA and TFIID: Friends of TBP drifting apart.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194604. doi: 10.1016/j.bbagrm.2020.194604. Epub 2020 Jul 14. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32673655 Review.
Cited by
-
Activator-Mediator binding regulates Mediator-cofactor interactions.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11283-8. doi: 10.1073/pnas.0914215107. Epub 2010 Jun 4. Proc Natl Acad Sci U S A. 2010. PMID: 20534441 Free PMC article.
-
Analysis of differential expression of Mediator subunit genes in Arabidopsis.Plant Signal Behav. 2012 Dec;7(12):1676-86. doi: 10.4161/psb.22438. Epub 2012 Oct 16. Plant Signal Behav. 2012. PMID: 23072992 Free PMC article.
-
The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation.Nat Rev Genet. 2010 Nov;11(11):761-72. doi: 10.1038/nrg2901. Epub 2010 Oct 13. Nat Rev Genet. 2010. PMID: 20940737 Free PMC article. Review.
-
Multiple faces of the SAGA complex.Curr Opin Cell Biol. 2010 Jun;22(3):374-82. doi: 10.1016/j.ceb.2010.03.005. Epub 2010 Apr 2. Curr Opin Cell Biol. 2010. PMID: 20363118 Free PMC article.
-
ATAC and Mediator coactivators form a stable complex and regulate a set of non-coding RNA genes.EMBO Rep. 2010 Jul;11(7):541-7. doi: 10.1038/embor.2010.75. Epub 2010 May 28. EMBO Rep. 2010. PMID: 20508642 Free PMC article.
References
-
- Andrau, J. C., L. van de Pasch, P. Lijnzaad, T. Bijma, M. G. Koerkamp, J. van de Peppel, M. Werner, and F. C. Holstege. 2006. Genome-wide location of the coactivator mediator: binding without activation and transient Cdk8 interaction on DNA. Mol. Cell 22179-192. - PubMed
-
- Baek, H. J., Y. K. Kang, and R. G. Roeder. 2006. Human Mediator enhances basal transcription by facilitating recruitment of TFIIB during preinitiation complex assembly. J. Biol. Chem. 28115172-15181. - PubMed
-
- Barlev, N. A., A. V. Emelyanov, P. Castagnino, P. Zegerman, A. J. Bannister, M. A. Sepulveda, F. Robert, L. Tora, T. Kouzarides, B. K. Birshtein, and S. L. Berger. 2003. A novel human Ada2 homologue functions with Gcn5 or Brg1 to coactivate transcription. Mol. Cell. Biol. 236944-6957. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
