Properties of the force exerted by filopodia and lamellipodia and the involvement of cytoskeletal components
- PMID: 17957254
- PMCID: PMC2034605
- DOI: 10.1371/journal.pone.0001072
Properties of the force exerted by filopodia and lamellipodia and the involvement of cytoskeletal components
Abstract
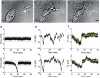
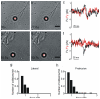
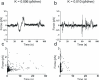
During neuronal differentiation, lamellipodia and filopodia explore the environment in search for the correct path to the axon's final destination. Although the motion of lamellipodia and filopodia has been characterized to an extent, little is known about the force they exert. In this study, we used optical tweezers to measure the force exerted by filopodia and lamellipodia with a millisecond temporal resolution. We found that a single filopodium exerts a force not exceeding 3 pN, whereas lamellipodia can exert a force up to 20 pN. Using metabolic inhibitors, we showed that no force is produced in the absence of actin polymerization and that development of forces larger than 3 pN requires microtubule polymerization. These results show that actin polymerization is necessary for force production and demonstrate that not only do neurons process information, but they also act on their environment exerting forces varying from tenths pN to tens of pN.
Conflict of interest statement
Figures



Similar articles
-
The role of myosin-II in force generation of DRG filopodia and lamellipodia.Sci Rep. 2015 Jan 19;5:7842. doi: 10.1038/srep07842. Sci Rep. 2015. PMID: 25598228 Free PMC article.
-
Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction.J Cell Biol. 1999 Sep 6;146(5):1097-106. doi: 10.1083/jcb.146.5.1097. J Cell Biol. 1999. PMID: 10477762 Free PMC article.
-
The Microtubule-Associated Protein Tau Mediates the Organization of Microtubules and Their Dynamic Exploration of Actin-Rich Lamellipodia and Filopodia of Cortical Growth Cones.J Neurosci. 2018 Jan 10;38(2):291-307. doi: 10.1523/JNEUROSCI.2281-17.2017. Epub 2017 Nov 22. J Neurosci. 2018. PMID: 29167405 Free PMC article.
-
Drebrin in Neuronal Migration and Axonal Growth.Adv Exp Med Biol. 2017;1006:141-155. doi: 10.1007/978-4-431-56550-5_9. Adv Exp Med Biol. 2017. PMID: 28865019 Review.
-
Lamellipodia and filopodia in metastasis and invasion.FEBS Lett. 2008 Jun 18;582(14):2102-11. doi: 10.1016/j.febslet.2008.03.039. Epub 2008 Apr 7. FEBS Lett. 2008. PMID: 18396168 Review.
Cited by
-
Current strategies and opportunities to manufacture cells for modeling human lungs.Adv Drug Deliv Rev. 2020;161-162:90-109. doi: 10.1016/j.addr.2020.08.005. Epub 2020 Aug 22. Adv Drug Deliv Rev. 2020. PMID: 32835746 Free PMC article.
-
Accounting for diffusion in agent based models of reaction-diffusion systems with application to cytoskeletal diffusion.PLoS One. 2011;6(9):e25306. doi: 10.1371/journal.pone.0025306. Epub 2011 Sep 23. PLoS One. 2011. PMID: 21966493 Free PMC article.
-
The Role of Rac1 in the Growth Cone Dynamics and Force Generation of DRG Neurons.PLoS One. 2016 Jan 14;11(1):e0146842. doi: 10.1371/journal.pone.0146842. eCollection 2016. PLoS One. 2016. PMID: 26766136 Free PMC article.
-
Manipulation of Axonal Outgrowth via Exogenous Low Forces.Int J Mol Sci. 2020 Oct 28;21(21):8009. doi: 10.3390/ijms21218009. Int J Mol Sci. 2020. PMID: 33126477 Free PMC article. Review.
-
Evolutionarily related small viral fusogens hijack distinct but modular actin nucleation pathways to drive cell-cell fusion.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2007526118. doi: 10.1073/pnas.2007526118. Proc Natl Acad Sci U S A. 2021. PMID: 33443166 Free PMC article.
References
-
- Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. - PubMed
-
- Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. - PubMed
-
- Gordon-Weeks PR. Microtubules and growth cone function. J Neurobiol. 2004;58:70–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

