Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes
- PMID: 17956583
- PMCID: PMC2219383
- DOI: 10.1111/j.1365-2249.2007.03521.x
Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes
Abstract
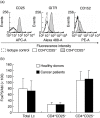
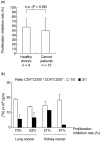
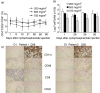
We determined the number and functional status of CD4+ CD25(high) regulatory T cells (Treg) in blood samples from patients with metastatic carcinoma, and evaluated their sensitivity to a single intravenous infusion of cyclophosphamide. Treg numbers were significantly higher in 49 patients with metastatic cancer (9.2% of CD4+ T cells) compared to 24 healthy donors (7.1%). These cells expressed the transcription factor forkhead box P3 (FoxP3), glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) and intracellular CD152, and demonstrated a suppressive activity in vitro against CD4+ CD25- autologous proliferation. At a single intravenous infusion, cyclophosphamide failed, in association with a non-specific immunotherapy by intratumoral bacille Calmette-Guérin (BCG), to modulate significantly Treg numbers or function. Metastatic cancer is associated with an expansion of peripheral blood CD4+ CD25(high) FoxP3+ GITR+ CD152+ Treg cells whose immunosuppressive properties do not differ from those of healthy subjects. Moreover, cyclophosphamide administration may not represent an optimal therapy to eliminate Treg, which further underlines the need to identify specific agents that would selectively deplete these cells.
Figures
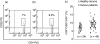
Similar articles
-
Increased frequency of CD4(+)CD25(high) Treg cells inhibit BCG-specific induction of IFN-gamma by CD4(+) T cells from TB patients.Tuberculosis (Edinb). 2007 Nov;87(6):526-34. doi: 10.1016/j.tube.2007.07.004. Epub 2007 Sep 11. Tuberculosis (Edinb). 2007. PMID: 17851131
-
CD4(+) CD25(low) GITR(+) cells: a novel human CD4(+) T-cell population with regulatory activity.Eur J Immunol. 2011 Aug;41(8):2269-78. doi: 10.1002/eji.201040943. Epub 2011 Jul 4. Eur J Immunol. 2011. PMID: 21557210
-
Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes.Crit Care Med. 2004 Nov;32(11):2329-31. doi: 10.1097/01.ccm.0000145999.42971.4b. Crit Care Med. 2004. PMID: 15640650
-
Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy.Curr Opin Investig Drugs. 2007 Dec;8(12):1002-8. Curr Opin Investig Drugs. 2007. PMID: 18058571 Review.
-
Immunomodulatory effects of cyclophosphamide and implementations for vaccine design.Semin Immunopathol. 2011 Jul;33(4):369-83. doi: 10.1007/s00281-011-0245-0. Epub 2011 May 25. Semin Immunopathol. 2011. PMID: 21611872 Review.
Cited by
-
Repurposing Infectious Diseases Vaccines Against Cancer.Front Oncol. 2021 May 13;11:688755. doi: 10.3389/fonc.2021.688755. eCollection 2021. Front Oncol. 2021. PMID: 34055652 Free PMC article. Review.
-
Cyclic adenosine monophosphate involvement in low-dose cyclophosphamide-reversed immune evasion in a mouse lymphoma model.Cell Mol Immunol. 2012 Nov;9(6):482-8. doi: 10.1038/cmi.2012.34. Epub 2012 Sep 24. Cell Mol Immunol. 2012. PMID: 23000689 Free PMC article.
-
T-regulatory lymphocytes in peripheral blood of gastric and colorectal cancer patients.World J Gastroenterol. 2011 Jan 21;17(3):343-8. doi: 10.3748/wjg.v17.i3.343. World J Gastroenterol. 2011. PMID: 21253393 Free PMC article.
-
Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity.Front Immunol. 2013 Dec 11;4:438. doi: 10.3389/fimmu.2013.00438. Front Immunol. 2013. PMID: 24376443 Free PMC article. Review.
-
A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer.Cancer Immunol Immunother. 2016 Feb;65(2):151-60. doi: 10.1007/s00262-015-1781-6. Epub 2016 Jan 4. Cancer Immunol Immunother. 2016. PMID: 26728480 Free PMC article. Clinical Trial.
References
-
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. - PubMed
-
- Morse MA, Clay TM, Mosca P, Lyerly HK. Immunoregulatory T cells in cancer immunotherapy. Expert Opin Biol Ther. 2002;2:827–34. - PubMed
-
- Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. - PubMed
-
- Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

