Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection
- PMID: 17942537
- PMCID: PMC2224348
- DOI: 10.1128/JVI.00927-07
Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection
Abstract
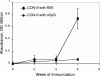
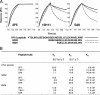
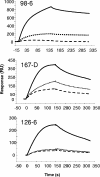
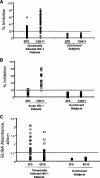
Two human monoclonal antibodies (MAbs) (2F5 and 4E10) against the human immunodeficiency virus type 1 (HIV-1) envelope g41 cluster II membrane proximal external region (MPER) broadly neutralize HIV-1 primary isolates. However, these antibody specificities are rare, are not induced by Env immunization or HIV-1 infection, and are polyspecific and also react with lipids such as cardiolipin or phosphatidylserine. To probe MPER anti-gp41 antibodies that are produced in HIV-1 infection, we have made two novel murine MAbs, 5A9 and 13H11, against HIV-1 gp41 envelope that partially cross-blocked 2F5 MAb binding to Env but did not neutralize HIV-1 primary isolates or bind host lipids. Competitive inhibition assays using labeled 13H11 MAb and HIV-1-positive patient plasma samples demonstrated that cluster II 13H11-blocking plasma antibodies were made in 83% of chronically HIV-1 infected patients and were acquired between 5 to 10 weeks after acute HIV-1 infection. Both the mouse 13H11 MAb and the three prototypic cluster II human MAbs (98-6, 126-6, and 167-D) blocked 2F5 binding to gp41 epitopes to variable degrees; the combination of 98-6 and 13H11 completely blocked 2F5 binding. These data provide support for the hypothesis that in some patients, B cells make nonneutralizing cluster II antibodies that may mask or otherwise down-modulate B-cell responses to immunogenic regions of gp41 that could be recognized by B cells capable of producing antibodies like 2F5.
Figures


Similar articles
-
The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes.J Immunol. 2007 Apr 1;178(7):4424-35. doi: 10.4049/jimmunol.178.7.4424. J Immunol. 2007. PMID: 17372000 Free PMC article.
-
In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth.J Virol. 2009 Apr;83(8):3617-25. doi: 10.1128/JVI.02631-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193787 Free PMC article.
-
The broadly neutralizing anti-human immunodeficiency virus type 1 4E10 monoclonal antibody is better adapted to membrane-bound epitope recognition and blocking than 2F5.J Virol. 2008 Sep;82(18):8986-96. doi: 10.1128/JVI.00846-08. Epub 2008 Jul 2. J Virol. 2008. PMID: 18596094 Free PMC article.
-
Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
-
The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design.Microbiol Mol Biol Rev. 2008 Mar;72(1):54-84, table of contents. doi: 10.1128/MMBR.00020-07. Microbiol Mol Biol Rev. 2008. PMID: 18322034 Free PMC article. Review.
Cited by
-
Nonneutralizing HIV-1 gp41 envelope cluster II human monoclonal antibodies show polyreactivity for binding to phospholipids and protein autoantigens.J Virol. 2011 Feb;85(3):1340-7. doi: 10.1128/JVI.01680-10. Epub 2010 Nov 24. J Virol. 2011. PMID: 21106741 Free PMC article.
-
Critical issues in mucosal immunity for HIV-1 vaccine development.J Allergy Clin Immunol. 2008 Jul;122(1):3-9; quiz 10-1. doi: 10.1016/j.jaci.2008.03.036. Epub 2008 May 12. J Allergy Clin Immunol. 2008. PMID: 18468671 Free PMC article. Review.
-
Importance of the membrane-perturbing properties of the membrane-proximal external region of human immunodeficiency virus type 1 gp41 to viral fusion.J Virol. 2008 Jun;82(11):5118-26. doi: 10.1128/JVI.00305-08. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353966 Free PMC article.
-
Anti-MPER antibodies with heterogeneous neutralization capacity are detectable in most untreated HIV-1 infected individuals.Retrovirology. 2014 Jun 7;11:44. doi: 10.1186/1742-4690-11-44. Retrovirology. 2014. PMID: 24909946 Free PMC article.
-
Determination of Binding Affinity of Antibodies to HIV-1 Recombinant Envelope Glycoproteins, Pseudoviruses, Infectious Molecular Clones, and Cell-Expressed Trimeric gp160 Using Microscale Thermophoresis.Cells. 2023 Dec 22;13(1):33. doi: 10.3390/cells13010033. Cells. 2023. PMID: 38201237 Free PMC article.
References
-
- Alam, S. M., C. A. Paleos, H-X. Liao, R. M. Scearce, J. Robinson, and B. F. Haynes. 2004. An inducible HIV-1 gp41 HR-2 peptide binding site on HIV-1 envelope gp120. AIDS Res. Hum. Retrovir. 20836-845. - PubMed
-
- Alam, S. M., M. McAdam, D. Boren, M. Rak, R. M. Scearce, F. Gao, Z. T. Camacho, D. Gewirth, G. Kelsoe, P. Chen, and B. F. Haynes. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to gp41 membrane proximal envelope epitopes. J. Immunol. 1784424-4435. - PMC - PubMed
-
- Barin, F., M. F. McLane, J. S. Allan, T. H. Lee, J. E. Groopman, and M. Essex. 1985. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science 2281094-1096. - PubMed
-
- Bowman, J. M. 1988. The prevention of Rh immunization. Transfus. Med. Rev. 2129-150. - PubMed
-
- Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

