Dopamine release in prefrontal cortex in response to beta-amyloid activation of alpha7 * nicotinic receptors
- PMID: 17935702
- PMCID: PMC2153437
- DOI: 10.1016/j.brainres.2007.08.079
Dopamine release in prefrontal cortex in response to beta-amyloid activation of alpha7 * nicotinic receptors
Abstract
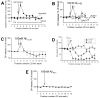
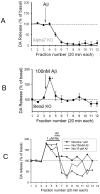
The levels of soluble beta-amyloid (Abeta) are correlated with symptom severity in Alzheimer's disease. Soluble Abeta has been shown to disrupt synaptic function and it has been proposed that accumulation of soluble Abeta triggers synapse loss over the course of the disease. Numerous studies indicate that soluble Abeta has multiple targets, one of which appears to be the nicotinic acetylcholine receptor, particularly for Abeta concentrations of pM to nM. Moreover, pM to nM soluble Abeta was found to increase presynaptic Ca(2+) levels, suggesting that it may have an impact on neurotransmitter release. In the present study, soluble Abeta was perfused into mouse prefrontal cortex and the effect on the release of dopamine outflow via microdialysis was assessed. In the presence of tetrodotoxin, Abeta(1-42) at 100 nM evoked the release of dopamine to approximately 170% of basal levels. The Abeta(1-42)-evoked dopamine release was sensitive to antagonists of alpha7 nicotinic receptors and was absent in mice harboring a null mutation for the alpha7 nicotinic subunit, but was intact in mice harboring a null mutation for the beta2 nicotinic subunit. The control peptide Abeta(40-1) was without effect. In contrast, Abeta(1-42) at 1-10 pM caused a profound but slowly developing decrease in dopamine outflow. These results suggest that Abeta alters dopamine release in mouse prefrontal cortex, perhaps involving distinct targets as it accumulates during Alzheimer's disease and leading to disruption of synaptic signaling.
Figures
Similar articles
-
Defining pre-synaptic nicotinic receptors regulated by beta amyloid in mouse cortex and hippocampus with receptor null mutants.J Neurochem. 2009 Jun;109(5):1452-8. doi: 10.1111/j.1471-4159.2009.06070.x. Epub 2009 Mar 28. J Neurochem. 2009. PMID: 19457164 Free PMC article.
-
Failure of nicotine-dependent enhancement of synaptic efficacy at Schaffer-collateral CA1 synapses of AD11 anti-nerve growth factor transgenic mice.Eur J Neurosci. 2006 Sep;24(5):1252-64. doi: 10.1111/j.1460-9568.2006.04996.x. Eur J Neurosci. 2006. PMID: 16987213
-
alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex.Eur J Neurosci. 2009 Feb;29(3):539-50. doi: 10.1111/j.1460-9568.2009.06613.x. Epub 2009 Jan 28. Eur J Neurosci. 2009. PMID: 19187266
-
Inhibitory effects of beta-amyloid on the nicotinic receptors which stimulate glutamate release in rat hippocampus: the glial contribution.Eur J Pharmacol. 2014 Jan 15;723:314-21. doi: 10.1016/j.ejphar.2013.11.011. Epub 2013 Nov 23. Eur J Pharmacol. 2014. PMID: 24275353
-
Glutamate-dopamine crosstalk in the rat prefrontal cortex is modulated by Alpha7 nicotinic receptors and potentiated by PNU-120596.J Mol Neurosci. 2010 Jan;40(1-2):172-6. doi: 10.1007/s12031-009-9232-5. Epub 2009 Aug 18. J Mol Neurosci. 2010. PMID: 19688191
Cited by
-
The interactions of dopamine and oxidative damage in the striatum of patients with neurodegenerative diseases.J Neurochem. 2020 Jan;152(2):235-251. doi: 10.1111/jnc.14898. Epub 2019 Nov 4. J Neurochem. 2020. PMID: 31613384 Free PMC article.
-
Amyloid β causes excitation/inhibition imbalance through dopamine receptor 1-dependent disruption of fast-spiking GABAergic input in anterior cingulate cortex.Sci Rep. 2018 Jan 10;8(1):302. doi: 10.1038/s41598-017-18729-5. Sci Rep. 2018. PMID: 29321592 Free PMC article.
-
The current agonists and positive allosteric modulators of α7 nAChR for CNS indications in clinical trials.Acta Pharm Sin B. 2017 Nov;7(6):611-622. doi: 10.1016/j.apsb.2017.09.001. Epub 2017 Oct 16. Acta Pharm Sin B. 2017. PMID: 29159020 Free PMC article. Review.
-
Nicotinic receptors, amyloid-beta, and synaptic failure in Alzheimer's disease.J Mol Neurosci. 2010 Jan;40(1-2):221-9. doi: 10.1007/s12031-009-9237-0. Epub 2009 Aug 19. J Mol Neurosci. 2010. PMID: 19690986 Review.
-
Alzheimer's Disease as a Membrane Disorder: Spatial Cross-Talk Among Beta-Amyloid Peptides, Nicotinic Acetylcholine Receptors and Lipid Rafts.Front Cell Neurosci. 2019 Jul 18;13:309. doi: 10.3389/fncel.2019.00309. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31379503 Free PMC article. Review.
References
-
- Arnsten AFT, Li B.-Mi. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57:1377–1384. - PubMed
-
- Ashenafi S, Fuente A, Criado JM, Riolobos AS, Heredia M, Yajeya J. β-amyloid peptide25-35 depresses excitatory synaptic transmission in the rat basolateral amygdale “in vitro”. Neurobiol. Aging. 2005;26:419–428. - PubMed
-
- Bell KA, O’Riordan KJ, Sweatt JD, Dineley KT. MAPK recruitment by β-amyloid in organotypic hippocampal slice cultures depends on physical state and exposure time. J. Neurochem. 2004;91:349–361. - PubMed
-
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J. Neurosci. Meth. 2001;105:133–141. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

