Low-dose antigen-experienced CD4+ T cells display reduced clonal expansion but facilitate an effective memory pool in response to secondary exposure
- PMID: 17916164
- PMCID: PMC2433333
- DOI: 10.1111/j.1365-2567.2007.02707.x
Low-dose antigen-experienced CD4+ T cells display reduced clonal expansion but facilitate an effective memory pool in response to secondary exposure
Abstract
The strength and duration of an antigenic signal at the time of initial stimulation were assumed to affect the development and response of effectors and memory cells to secondary stimulation with the same antigen. To test this assumption, we used T-cell receptor (TCR)-transgenic CD4+ T cells that were stimulated in vitro with various antigen doses. The primary effector CD4+ T cells generated in response to low-dose antigen in vitro exhibited reduced clonal expansion upon secondary antigenic exposure after adoptive transfer to hosts. However, the magnitude of their contraction was much smaller than both those generated by high-dose antigen stimulation and by naïve CD4+ T cells, resulting in higher numbers of antigen-specific CD4+ T cells remaining until the memory stage. Moreover, secondary effectors and memory cells developed by secondary antigen exposure were not functionally impaired. In hosts given the low-dose antigen-experienced CD4+ T cells, we also observed accelerated recall responses upon injection of antigen-bearing antigen-presenting cells. These results suggest that primary TCR stimulation is important for developing optimal effectors during initial antigen exposure to confer long-lasting memory CD4+ T cells in response to secondary exposure.
Figures
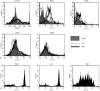
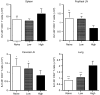

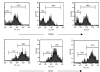

Similar articles
-
Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host.J Immunol. 2000 May 1;164(9):4551-7. doi: 10.4049/jimmunol.164.9.4551. J Immunol. 2000. PMID: 10779756
-
Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment. II. The memory T cell response is diminished.Mech Ageing Dev. 2004 Jan;125(1):59-68. doi: 10.1016/j.mad.2003.10.003. Mech Ageing Dev. 2004. PMID: 14706238
-
Antigen-specific CD4+ effector T cells: analysis of factors regulating clonal expansion and cytokine production: clonal expansion and cytokine production by CD4+ effector T cells.Biochem Biophys Res Commun. 2009 Mar 20;380(4):742-7. doi: 10.1016/j.bbrc.2009.01.123. Epub 2009 Jan 25. Biochem Biophys Res Commun. 2009. PMID: 19338745
-
The effector to memory transition of CD4 T cells.Immunol Res. 2008;40(2):114-27. doi: 10.1007/s12026-007-8004-y. Immunol Res. 2008. PMID: 18213525 Review.
-
CD4 T-cell memory can persist in the absence of class II.Philos Trans R Soc Lond B Biol Sci. 2000 Mar 29;355(1395):407-11. doi: 10.1098/rstb.2000.0581. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10794062 Free PMC article. Review.
Cited by
-
B cells are sufficient to prime the dominant CD4+ Tfh response to Plasmodium infection.J Exp Med. 2020 Feb 3;217(2):e20190849. doi: 10.1084/jem.20190849. J Exp Med. 2020. PMID: 31748243 Free PMC article.
-
Evaluation of in vivo T cell kinetics: use of heavy isotope labelling in type 1 diabetes.Clin Exp Immunol. 2013 Jun;172(3):363-74. doi: 10.1111/cei.12064. Clin Exp Immunol. 2013. PMID: 23600824 Free PMC article.
-
Striking a Balance-Cellular and Molecular Drivers of Memory T Cell Development and Responses to Chronic Stimulation.Front Immunol. 2019 Jul 17;10:1595. doi: 10.3389/fimmu.2019.01595. eCollection 2019. Front Immunol. 2019. PMID: 31379821 Free PMC article. Review.
-
Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials.Viruses. 2010 Feb 1;2(2):435-467. doi: 10.3390/v2020435. Viruses. 2010. PMID: 20407589 Free PMC article.
-
Presentation of high antigen-dose by splenic B220(lo) B cells fosters a feedback loop between T helper type 2 memory and antibody isotype switching.Immunology. 2016 Apr;147(4):464-75. doi: 10.1111/imm.12579. Epub 2016 Jan 28. Immunology. 2016. PMID: 26749165 Free PMC article.
References
-
- Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–67. - PubMed
-
- Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. - PubMed
-
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. - PubMed
-
- van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–84. - PubMed
-
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

