Tumor necrosis factor and stroke: role of the blood-brain barrier
- PMID: 17913328
- PMCID: PMC2190541
- DOI: 10.1016/j.pneurobio.2007.07.008
Tumor necrosis factor and stroke: role of the blood-brain barrier
Abstract
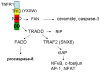
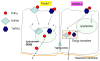
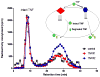
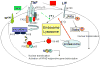
The progression and outcome of stroke is affected by the intricate relationship between the blood-brain barrier (BBB) and tumor necrosis factor alpha (TNFalpha). TNFalpha crosses the intact BBB by a receptor-mediated transport system that is upregulated by CNS trauma and inflammation. In this review, we discuss intracellular trafficking and transcytosis of TNFalpha, regulation of TNFalpha transport after stroke, and the effects of TNFalpha on stroke preconditioning. TNFalpha can activate cytoprotective pathways by pretreatment or persistent exposure to low doses. This explains the paradoxical observation that transport of this proinflammatory cytokine improves the survival and function of hypoxic cells and of mice with stroke. The dual effects of TNFalpha may be related to differential regulation of TNFalpha trafficking downstream to TNFR1 and TNFR2 receptors. As we better understand how peripheral TNFalpha affects its own transport and modulates neuroregeneration, we may be in a better position to pharmacologically manipulate its regulatory transport system to treat stroke.
Figures
Similar articles
-
Stroke upregulates TNFalpha transport across the blood-brain barrier.Exp Neurol. 2006 Mar;198(1):222-33. doi: 10.1016/j.expneurol.2005.11.020. Epub 2006 Jan 17. Exp Neurol. 2006. PMID: 16412421
-
TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice.Exp Neurol. 2002 Apr;174(2):193-200. doi: 10.1006/exnr.2002.7871. Exp Neurol. 2002. PMID: 11922661
-
Upregulation of the transport system for TNFalpha at the blood-brain barrier.Arch Physiol Biochem. 2001 Oct;109(4):350-3. doi: 10.1076/apab.109.4.350.4238. Arch Physiol Biochem. 2001. PMID: 11935370 Review.
-
Differential roles of TNFα-TNFR1 and TNFα-TNFR2 in the differentiation and function of CD4+Foxp3+ induced Treg cells in vitro and in vivo periphery in autoimmune diseases.Cell Death Dis. 2019 Jan 10;10(1):27. doi: 10.1038/s41419-018-1266-6. Cell Death Dis. 2019. PMID: 30631042 Free PMC article.
-
Angioneurins - Key regulators of blood-brain barrier integrity during hypoxic and ischemic brain injury.Prog Neurobiol. 2019 Jul;178:101611. doi: 10.1016/j.pneurobio.2019.03.004. Epub 2019 Apr 7. Prog Neurobiol. 2019. PMID: 30970273 Review.
Cited by
-
Innovative in vivo rat model for global cerebral hypoxia: a new approach to investigate therapeutic and preventive drugs.Front Physiol. 2024 Feb 9;15:1293247. doi: 10.3389/fphys.2024.1293247. eCollection 2024. Front Physiol. 2024. PMID: 38405120 Free PMC article.
-
Shuxuetong injection protects cerebral microvascular endothelial cells against oxygen-glucose deprivation reperfusion.Neural Regen Res. 2019 May;14(5):783-793. doi: 10.4103/1673-5374.249226. Neural Regen Res. 2019. PMID: 30688264 Free PMC article.
-
Cerebral Small Vessel Disease: Neuroimaging Features, Biochemical Markers, Influencing Factors, Pathological Mechanism and Treatment.Front Neurol. 2022 Jun 14;13:843953. doi: 10.3389/fneur.2022.843953. eCollection 2022. Front Neurol. 2022. PMID: 35775047 Free PMC article. Review.
-
Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets.Curr Med Chem. 2014;21(18):2076-97. doi: 10.2174/0929867321666131228205146. Curr Med Chem. 2014. PMID: 24372209 Free PMC article. Review.
-
Dual Functions of Microglia in Ischemic Stroke.Neurosci Bull. 2019 Oct;35(5):921-933. doi: 10.1007/s12264-019-00388-3. Epub 2019 May 6. Neurosci Bull. 2019. PMID: 31062335 Free PMC article. Review.
References
-
- Abbruscato TJ, Lopez S, Roder K, Paulson J. Regulation of blood-brain barrier Na,K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions. J Pharmacol Exp Ther. 2004;310:459–468. - PubMed
-
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Krönke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. - PubMed
-
- Atochin DN, Murciano JC, Gursoy-Ozdemir Y, Krasik T, Noda F, Ayata C, Dunn AK, Moskowitz MA, Huang PL, Muzykantov VR. Mouse model of microembolic stroke and reperfusion. Stroke. 2004;35:2177–2182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

