Both TRIM5alpha and TRIMCyp have only weak antiviral activity in canine D17 cells
- PMID: 17892575
- PMCID: PMC2064933
- DOI: 10.1186/1742-4690-4-68
Both TRIM5alpha and TRIMCyp have only weak antiviral activity in canine D17 cells
Abstract
Background: TRIM5alpha, which is expressed in most primates and the related TRIMCyp, which has been found in one of the New World monkey species, are antiviral proteins of the TRIM5 family that are able to intercept incoming retroviruses early after their entry into cells. The mechanism of action has been partially elucidated for TRIM5alpha, which seems to promote premature decapsidation of the restricted retroviruses. In addition, through its N-terminal RING domain, TRIM5alpha may sensitize retroviruses to proteasome-mediated degradation. TRIM5alpha-mediated restriction requires a physical interaction with the capsid protein of targeted retroviruses. It is unclear whether other cellular proteins are involved in the inhibition mediated by TRIM5alpha and TRIMCyp. A previous report suggested that the inhibition of HIV-1 by the rhesus macaque orthologue of TRIM5alpha was inefficient in the D17a canine cell line, suggesting that the cellular environment was important for the restriction mechanism. Here we investigated further the behavior of TRIM5alpha and TRIMCyp in the D17 cells.
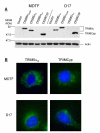
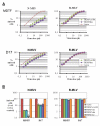
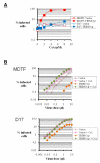
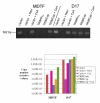
Results: We found that the various TRIM5alpha orthologues studied (human, rhesus macaque, African green monkey) as well as TRIMCyp had poor antiviral activity in the D17 cells, despite seemingly normal expression levels and subcellular distribution. Restriction of both HIV-1 and the distantly related N-tropic murine leukemia virus (N-MLV) was low in D17 cells. Both TRIM5alpharh and TRIMCyp promoted early HIV-1 decapsidation in murine cells, but weak levels of restriction in D17 cells correlated with the absence of accelerated decapsidation in these cells and also correlated with normal levels of cDNA synthesis. Fv1, a murine restriction factor structurally unrelated to TRIM5alpha, was fully functional in D17 cells, showing that the loss of activity was specific to TRIM5alpha/TRIMCyp.
Conclusion: We show that D17 cells provide a poor environment for the inhibition of retroviral replication by proteins of the TRIM5 family. Because both TRIM5alpha and TRIMCyp are poorly active in these cells, despite having quite different viral target recognition domains, we conclude that a step either upstream or downstream of target recognition is impaired. We speculate that an unknown factor required for TRIM5alpha and TRIMCyp activity is missing or inadequately expressed in D17 cells.
Figures



Similar articles
-
Implication of TRIM alpha and TRIMCyp in interferon-induced anti-retroviral restriction activities.Retrovirology. 2008 Jul 9;5:59. doi: 10.1186/1742-4690-5-59. Retrovirology. 2008. PMID: 18613956 Free PMC article.
-
Disruption of human TRIM5alpha antiviral activity by nonhuman primate orthologues.J Virol. 2005 Jun;79(12):7883-8. doi: 10.1128/JVI.79.12.7883-7888.2005. J Virol. 2005. PMID: 15919943 Free PMC article.
-
Association of TRIMCyp and TRIM5α from assam macaques leads to a functional trade-off between HIV-1 and N-MLV inhibition.Sci China Life Sci. 2018 Aug;61(8):954-965. doi: 10.1007/s11427-018-9295-y. Epub 2018 Apr 26. Sci China Life Sci. 2018. PMID: 29705873
-
[The primate TRIMCyp fusion genes and mechanism of restricting retroviruses replication].Dongwuxue Yanjiu. 2012 Feb;33(1):99-107. doi: 10.3724/SP.J.1141.2012.01099. Dongwuxue Yanjiu. 2012. PMID: 22345017 Review. Chinese.
-
Recent insights into the mechanism and consequences of TRIM5α retroviral restriction.AIDS Res Hum Retroviruses. 2011 Mar;27(3):231-8. doi: 10.1089/AID.2010.0367. AIDS Res Hum Retroviruses. 2011. PMID: 21247355 Free PMC article. Review.
Cited by
-
Preclinical Assessment of Mutant Human TRIM5α as an Anti-HIV-1 Transgene.Hum Gene Ther. 2015 Oct;26(10):664-79. doi: 10.1089/hum.2015.059. Epub 2015 Aug 6. Hum Gene Ther. 2015. PMID: 26076730 Free PMC article.
-
TRIM5alpha and TRIMCyp form apparent hexamers and their multimeric state is not affected by exposure to restriction-sensitive viruses or by treatment with pharmacological inhibitors.Retrovirology. 2009 Nov 3;6:100. doi: 10.1186/1742-4690-6-100. Retrovirology. 2009. PMID: 19886997 Free PMC article.
-
Cytoplasmic dynein promotes HIV-1 uncoating.Viruses. 2014 Nov 4;6(11):4195-211. doi: 10.3390/v6114195. Viruses. 2014. PMID: 25375884 Free PMC article.
-
Delaying reverse transcription does not increase sensitivity of HIV-1 to human TRIM5α.PLoS One. 2013;8(1):e52434. doi: 10.1371/journal.pone.0052434. Epub 2013 Jan 8. PLoS One. 2013. PMID: 23320071 Free PMC article.
-
Molecular evolution of the antiretroviral TRIM5 gene.Immunogenetics. 2009 Mar;61(3):163-76. doi: 10.1007/s00251-009-0358-y. Epub 2009 Feb 24. Immunogenetics. 2009. PMID: 19238338 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

