Novel membrane-bound eIF2alpha kinase in the flagellar pocket of Trypanosoma brucei
- PMID: 17873083
- PMCID: PMC2168417
- DOI: 10.1128/EC.00249-07
Novel membrane-bound eIF2alpha kinase in the flagellar pocket of Trypanosoma brucei
Abstract
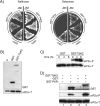
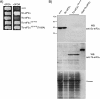
Translational control mediated by phosphorylation of the alpha subunit of the eukaryotic initiation factor 2 (eIF2alpha) is central to stress-induced programs of gene expression. Trypanosomatids, important human pathogens, display differentiation processes elicited by contact with the distinct physiological milieu found in their insect vectors and mammalian hosts, likely representing stress situations. Trypanosoma brucei, the agent of African trypanosomiasis, encodes three potential eIF2alpha kinases (TbeIF2K1 to -K3). We show here that TbeIF2K2 is a transmembrane glycoprotein expressed both in procyclic and in bloodstream forms. The catalytic domain of TbeIF2K2 phosphorylates yeast and mammalian eIF2alpha at Ser51. It also phosphorylates the highly unusual form of eIF2alpha found in trypanosomatids specifically at residue Thr169 that corresponds to Ser51 in other eukaryotes. T. brucei eIF2alpha, however, is not a substrate for GCN2 or PKR in vitro. The putative regulatory domain of TbeIF2K2 does not share any sequence similarity with known eIF2alpha kinases. In both procyclic and bloodstream forms TbeIF2K2 is mainly localized in the membrane of the flagellar pocket, an organelle that is the exclusive site of exo- and endocytosis in these parasites. It can also be detected in endocytic compartments but not in lysosomes, suggesting that it is recycled between endosomes and the flagellar pocket. TbeIF2K2 location suggests a relevance in sensing protein or nutrient transport in T. brucei, an organism that relies heavily on posttranscriptional regulatory mechanisms to control gene expression in different environmental conditions. This is the first membrane-associated eIF2alpha kinase described in unicellular eukaryotes.
Figures


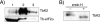




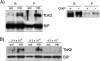
Similar articles
-
Phosphorylation of eIF2α on Threonine 169 is not required for Trypanosoma brucei cell cycle arrest during differentiation.Mol Biochem Parasitol. 2016 Jan-Feb;205(1-2):16-21. doi: 10.1016/j.molbiopara.2016.03.004. Epub 2016 Mar 17. Mol Biochem Parasitol. 2016. PMID: 26996431 Free PMC article.
-
Clathrin-dependent targeting of receptors to the flagellar pocket of procyclic-form Trypanosoma brucei.Eukaryot Cell. 2004 Aug;3(4):1004-14. doi: 10.1128/EC.3.4.1004-1014.2004. Eukaryot Cell. 2004. PMID: 15302833 Free PMC article.
-
Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the Trypanosoma brucei Flagellar membrane.Eukaryot Cell. 2014 Aug;13(8):1064-76. doi: 10.1128/EC.00019-14. Epub 2014 May 30. Eukaryot Cell. 2014. PMID: 24879126 Free PMC article.
-
The trypanosome flagellar pocket.Nat Rev Microbiol. 2009 Nov;7(11):775-86. doi: 10.1038/nrmicro2221. Epub 2009 Oct 6. Nat Rev Microbiol. 2009. PMID: 19806154 Review.
-
Flagellar membrane proteins in kinetoplastid parasites.IUBMB Life. 2015 Sep;67(9):668-76. doi: 10.1002/iub.1411. Epub 2015 Aug 25. IUBMB Life. 2015. PMID: 26599841 Free PMC article. Review.
Cited by
-
Enzyme Activity Assays for Protein Kinases: Strategies to Identify Active Substrates.Curr Drug Discov Technol. 2016;13(1):2-15. doi: 10.2174/1570163813666160115125930. Curr Drug Discov Technol. 2016. PMID: 26768716 Free PMC article. Review.
-
A membrane-bound eIF2 alpha kinase located in endosomes is regulated by heme and controls differentiation and ROS levels in Trypanosoma cruzi.PLoS Pathog. 2015 Feb 6;11(2):e1004618. doi: 10.1371/journal.ppat.1004618. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25658109 Free PMC article.
-
A GCN2-Like eIF2α Kinase (LdeK1) of Leishmania donovani and Its Possible Role in Stress Response.PLoS One. 2016 Jun 1;11(6):e0156032. doi: 10.1371/journal.pone.0156032. eCollection 2016. PLoS One. 2016. PMID: 27248816 Free PMC article.
-
Evolutionary conservation and diversification of the translation initiation apparatus in trypanosomatids.Comp Funct Genomics. 2012;2012:813718. doi: 10.1155/2012/813718. Epub 2012 Jul 8. Comp Funct Genomics. 2012. PMID: 22829751 Free PMC article.
-
Blocking variant surface glycoprotein synthesis in Trypanosoma brucei triggers a general arrest in translation initiation.PLoS One. 2009 Oct 26;4(10):e7532. doi: 10.1371/journal.pone.0007532. PLoS One. 2009. PMID: 19855834 Free PMC article.
References
-
- Alexander, D. L., K. J. Schwartz, A. E. Balber, and J. D. Bangs. 2002. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 115:3253-3263. - PubMed
-
- Bangs, J. D., L. Uyetake, M. J. Brickman, A. E. Balber, and J. C. Boothroyd. 1993. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei: divergent ER retention signals in a lower eukaryote. J. Cell Sci. 105:1101-1113. - PubMed
-
- Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, et al. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309:416-422. - PubMed
-
- Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. - PubMed
-
- Brecht, M., and M. Parsons. 1998. Changes in polysome profiles accompany trypanosome development. Mol. Biochem. Parasitol. 97:189-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

