Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells
- PMID: 17827281
- PMCID: PMC1986610
- DOI: 10.1073/pnas.0703767104
Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells
Abstract
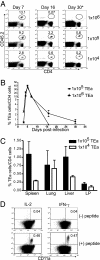
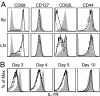
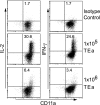
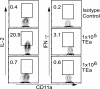
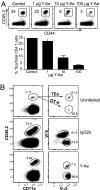
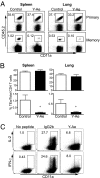
The factors involved in the differentiation of memory CD4 T cells from naïve precursors are poorly understood. We developed a system to examine the effect of increased competition for antigen by CD4 T cells on the generation of memory in response to infection with a recombinant vesicular stomatitis virus. Competition was initially regulated by increasing the precursor frequency of adoptively transferred naïve T cell antigen receptor transgenic CD4 T cells. Despite robust proliferation at high precursor frequencies, memory CD4 T cells did not develop, whereas decreasing the input number of naïve CD4 T cells promoted memory development after infection. The lack of memory development was linked to reduced blastogenesis and poor effector cell induction, but not to initial recruitment or proliferation of antigen-specific CD4 T cells. To prove that availability of antigen alone could regulate memory CD4 T cell development, we used treatment with an mAb specific for the epitope recognized by the transferred CD4 T cells. At high doses, this mAb effectively inhibited the antigen-specific CD4 T cell response. However, at a very low dose of mAb, primary CD4 T cell expansion was unaffected, although memory development was dramatically reduced. Moreover, the induction of effector function was concomitantly inhibited. Thus, competition for antigen during CD4 T cell priming is a major contributing factor to the development of the memory CD4 T cell pool.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The phenotype and survival of antigen-stimulated transgenic CD4 T cells in vivo: the influence of persisting antigen.Int Immunol. 2006 Apr;18(4):515-23. doi: 10.1093/intimm/dxh392. Epub 2006 Feb 15. Int Immunol. 2006. PMID: 16481344
-
Blockade of CTLA-4 decreases the generation of multifunctional memory CD4+ T cells in vivo.J Immunol. 2011 May 15;186(10):5580-9. doi: 10.4049/jimmunol.1003381. Epub 2011 Apr 8. J Immunol. 2011. PMID: 21478403
-
Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen.J Immunol. 1993 Apr 15;150(8 Pt 1):3119-30. J Immunol. 1993. PMID: 8096850
-
Effect of age on naive CD4 responses: impact on effector generation and memory development.Springer Semin Immunopathol. 2002;24(1):53-60. doi: 10.1007/s00281-001-0095-2. Springer Semin Immunopathol. 2002. PMID: 11974581 Review. No abstract available.
-
A role for inflammatory cytokines in the productive activation of antigen-specific CD4+ T-cells.Agents Actions Suppl. 1998;49:23-31. doi: 10.1007/978-3-0348-8857-8_5. Agents Actions Suppl. 1998. PMID: 9426825 Review.
Cited by
-
CD49b/CD69-Dependent Generation of Resting T Helper Cell Memory.Front Immunol. 2013 Jul 10;4:183. doi: 10.3389/fimmu.2013.00183. eCollection 2013. Front Immunol. 2013. PMID: 23847623 Free PMC article.
-
Diversity in T cell memory: an embarrassment of riches.Immunity. 2009 Dec 18;31(6):859-71. doi: 10.1016/j.immuni.2009.11.007. Immunity. 2009. PMID: 20064446 Free PMC article. Review.
-
Control of alpha4beta7 integrin expression and CD4 T cell homing by the beta1 integrin subunit.J Immunol. 2010 Mar 1;184(5):2458-67. doi: 10.4049/jimmunol.0902407. Epub 2010 Jan 29. J Immunol. 2010. PMID: 20118278 Free PMC article.
-
ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes.Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):10961-6. doi: 10.1073/pnas.0801496105. Epub 2008 Jul 30. Proc Natl Acad Sci U S A. 2008. PMID: 18667699 Free PMC article.
-
A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection.J Immunol. 2008 Jun 1;180(11):7203-11. doi: 10.4049/jimmunol.180.11.7203. J Immunol. 2008. PMID: 18490719 Free PMC article.
References
-
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. J Immunol. 2000;165:6833–6839. - PubMed
-
- Van Stipdonk MJ, Lemmens EE, Schoenberger SP. Nat Immunol. 2001;2:423–429. - PubMed
-
- Van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Nat Immunol. 2003;4:361–365. - PubMed
-
- Iezzi G, Karjalainen K, Lanzavecchia A. Immunity. 1998;8:89–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

